Abstract
Experimental autoimmune encephalomyelitis (EAE) is a T-cell-mediated autoimmune central nervous system disease characterized by inflammation with oxidative stress. The aim of this study was to evaluate an anti-inflammatory effect of Ishige okamurae on EAE-induced paralysis in rats. An ethanolic extract of I. okamurae significantly delayed the first onset and reduced the duration and severity of hind-limb paralysis. The neuropathological and immunohistochemical findings in the spinal cord were in agreement with these clinical results. T-cell proliferation assay revealed that the ethyl-acetate fraction of I. okamurae suppressed the proliferation of myelin basic protein reactive T cells from EAE affected rats. Flow cytometric analysis showed TCRαβ+ T cells was significantly reduced in the spleen of EAE rats with I. okamurae treatment with concurrent decrease of inflammatory mediators including tumor necrosis factor-α and cyclooxygenase-2. Collectively, it is postulated that I. okamurae ameliorates EAE paralysis with suppression of T-cell proliferation as well as decrease of pro-inflammatory mediators as far as rat EAE is concerned.
Experimental autoimmune encephalomyelitis (EAE), a model of human demyelinating multiple sclerosis (MS), is mediated by the infiltration of autoimmune CD4+/Th1 cells and macrophages, with subsequent activation of microglia and astrocytes [1]. Th1 cytokines, interferon-gamma and tumor necrosis factor (TNF)-α have been considered to play a crucial role in the development of EAE [2]. EAE-inducing T cells have been well characterized and shown to be neuroantigen-reactive CD4+/TCRαβ+ T cells [3]. With regard to MS, it is thought that TCRαβ+ T cells are involved in the initiation of MS [3]. The inflammatory response leads to the generation of oxygen- and nitrogen-free radicals as well as pro-inflammatory cytokines, which contribute to the development and progression of MS [4]. In MS [5] and EAE [6], inflammatory cells and glial cells produce free radicals and excessive reactive oxidative stress derived from macrophage triggers oxidative damages, aggravates demyelination, axonal damage, and neuronal cell death [456]. Therefore, antioxidants have been used to ameliorate central nervous system (CNS) damage such as EAE and other neurodegenerative disease associated with oxidative stress.
Ishige okamurae (phylum Phaeophyta, class Phaeophyceae, order Chordariales, family Ishigeaceae; I. okamurae), a brown algae, is an edible seaweed [7]. The ethanolic extract of I. okamurae exerts an anti-inflammatory effect by inhibiting nuclear factor κB transcription factor in RAW 264.7 cells [8], and its phlorotannin component diphlorethohydroxycarmalol has antioxidant in free radical mediated oxidative systems [910], neuroprotective against H2O2-induced oxidative stress in murine hippocampal neuronal cells [11], and radioprotective effects, the latter being mediated by free-radical scavenging activities [1213]. However, little is known whether I. okamurae ameliorate autoimmune CNS inflammation in animal models. The aim of this study was to evaluate whether I. okamurae attenuates autoimmune neuro-inflammation in in rat EAE model of MS.
Lewis rats (Harlan, Indianapolis, IN, USA) were bred in our animal facility. Rats of both sexes (7–8 weeks old; 160–200 g) were used in this study. All experiments were performed in accordance with accepted ethical guidelines and conformed to current international laws and policies (NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85–23, 1985, revised 1996). All experiments were performed in accordance with the ethical guidelines by Jeju National University Guide for the Care and Use of Laboratory Animals (permission No. 2014-0028).
The footpads of both hind feet of rats in the EAE group were injected with 100 µl of an emulsion containing equal parts of bovine myelin basic protein (MBP; 1 mg/ml) and complete Freund's adjuvant, supplemented with Mycobacterium tuberculosis H37Ra (5 mg/ml, Difco, Detroit, MI, USA). After immunization, the rats were observed daily for clinical signs of EAE. The progression of EAE was divided into eight clinical stages: grade 0 (G.0), no sign; G.0.5, mild floppy tail; G.1, complete floppy tail; G.2, mild paraparesis; G.3, severe paraparesis; G.4, tetraparesis; G.5, moribund condition or death; and R.0, recovery.
I. okamurae was collected from the coast of Seongsan, Jeju Island, in July 2005. A voucher specimen (AP-055) was deposited at Jeju Bio-Industry Development Center, Hi-Tech Industry Development Institute, Jeju, Korea. The samples were washed three times with water to remove surface salt, epiphytes, and sand, and carefully rinsed with fresh water.
A shade-dried whole I. okamurae (500 g) plant was extracted with 70% aqueous ethanol with stirring for 2 days at room temperature. The filtrate was concentrated under reduced pressure and lyophilized to powder. The powdered extract (76.9 g) was suspended in water (1.0 l) for use in animal experiments. The I. okamurae ethanolic extract was successively partitioned with n-hexane, ethyl acetate, and n-butanol (Junsei Chemical, Osaka, Japan).
Rats with EAE were intraperitoneally administered 1 (n=5), 10 (n=5), or 50 mg/kg (n=4) of I. okamurae daily from 1 day before immunization to 14 days post-immunization (PI) [14]. Control rats received vehicle (saline, n=8) according to the same protocol. Immunized rats were observed daily for clinical signs of EAE. EAE progression in I. okamurae-treated and vehicle-treated control rats was compared by Student's unpaired, two-tailed t-test. A value of P<0.05 was considered indicative of statistical significance.
T-cell proliferation assays were performed as described previously [14]. Briefly, spleen mononuclear cells (MNCs) from normal and EAE-affected rats (n=3 per group) at day 12 PI were dissociated and suspended in Dulbecco's modified Eagle's medium (DMEM; Gibco, Paisley, UK) supplemented with 1% (v/v) minimum essential medium (Gibco), 2 mmol glutamine (Flow Laboratory, Irvine, CA, USA), 50 IU/ml penicillin, 50 mg/ml streptomycin, and 10% (v/v) fetal calf serum (Gibco). MNCs were isolated and incubated (4×105) with 200 µl of DMEM in 96-well, round-bottomed microtiter plates (Nunc, Copenhagen, Denmark). MBP (Sigma, St. Louis, MO, USA) was next added to a final concentration of 10 µg/ml. Following incubation for 4 days, the cells were pulsed for 18 hours with 10 µl volumes containing 1 µCi of 3H-methylthymidine (specific activity, 42 Ci/mmol; Amersham, Arlington Heights, IL, USA). The cells were harvested on glass fiber filters, and thymidine incorporation was assayed.
To analyze TCR αβ+ T cells, single cell suspensions of spleen were prepared and incubated with R73 and then with fluorescein isothiocyanate-conjugated horse anti-mouse IgG (Sigma-Aldrich). Ten thousand cells were analyzed in each sample using a FACSCalibur (BD Biosciences, Becton, NJ, USA). All samples were analyzed on a WinMDI 2.8 using Diva software.
Total RNAs of spinal cords were extracted using the TRIzol RNA Isolation Reagent (Life Technologies, Carlsbad, CA, USA). Purified RNA was transcribed into cDNA using 5× First Strand cDNA Synthesis Master Mix (CellSafe, Yongin, Korea) according to the manufacturer's protocol.
Quantitative real-time polymerase chain reaction (PCR) was performed using the QuantiSpeed SYBR No-Rox Mix (Philekorea Co., Ltd., Seoul, Korea) according to the manufacturer's protocol. The primer sequences were as follows: glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward 5′-CAG CGC ATA CCA CTT CAG C-3′ and reverse 5′-ACC ATG GAG CAT CCC AAG-3′; cyclooxygenase (COX)-2, forward 5′-CGG AGG AGA AGT GGG GTT TA-3′ and reverse 5′-TGG GAG GCA CTT GCG TTG AT-3′; TNF-α, forward 5′-CGT CGT AGC AAA CCA CCA AG-3′ and reverse 5′-CAC AGA GCA ATG ACT CCA AA-3′. PCR reactions were performed using the Mic Real Time PCR Cycler (Biomolecular System, Potts Point, Australia). The PCR protocol consisted of 40 cycles of denaturation at 95℃ for 15 seconds, followed by 60℃ for 30 seconds to allow for extension and amplification of the target sequence. The relative expression levels of TNF-α and COX-2 were normalized to that of GAPDH using the 2−ΔΔCT method.
Three rats per group were sacrificed at day 28 PI under ether anesthesia, and the spinal cords were separated and dissected. Spinal cord specimens were embedded in paraffin after being fixed in 4% paraformaldehyde in phosphate-buffered saline (pH 7.4). Paraffin sections were subjected to hematoxylin and eosin staining and immunohistochemistry using routine procedures [15].
To evaluate microglial activation, spinal-cord tissues were immunostained for ionized calcium-binding protein (Iba-1, 1 µg/ml, Wako Pure Chemical Industries, Ltd., Osaka, Japan) using a Vector Elite ABC Kit (Vector laboratory, Birmingham, CA, USA), and the area of positivity was analyzed as reported previously [16].
I. okamurae (1, 10, or 50 mg/kg) was administered to rats intraperitoneally from 1 day before immunization to 14 days PI (Table 1). The first onset of EAE paralysis was significantly delayed in I. okamurae-treated rats (10 mg/kg, 15.7±1.2 days; 50 mg/kg, 16.3±0.7 days) in comparison with vehicle-treated control rats (11.5±1.0 days, P<0.001). Furthermore, I. okamurae treatment significantly reduced the duration of paralysis (10 mg/kg, 4.0±1 days; 50 mg/kg, 3.8±1.5 days) compared with vehicle (6.3±1.5 days, all P<0.001). However, the course of EAE paralysis in the I. okamurae (1 mg/kg) treated rats was not significantly different from that of the vehicle-treated control rats.
There was no inflammatory cell infiltration in the spinal cord tissues of normal control rats (Fig. 1A, D). Many inflammatory cells were detected in the spinal-cord parenchyma of vehicle-treated EAE rats (arrowheads in Fig. 1B; higher-magnification view in Fig. 1E). In contrast, the number of inflammatory cells was reduced in the spinal cords of rats treated with 10 mg/kg I. okamurae (arrowheads in Fig. 1C; higher magnification view in Fig. 1F).
The spinal cords of I. okamurae-treated rats contained fewer Iba-1-positive microglia/macrophages than did those of vehicle-treated control rats (Fig. 2). The area of ramified Iba-1-positive microglia (Fig. 2A) was 4.62±0.18% in normal control rats (Fig. 2D). The number of Iba-1-positive cells in the spinal cords of vehicle-treated rats (Fig. 2B) was markedly greater than that in normal control (Fig. 2A) and I. okamurae-treated (Fig. 2C) rats. The Iba-1-positive area in I. okamurae-treated rats (13.28±2.02%, P<0.05 vs. vehicle-treated EAE rats) was significantly reduced compared to that in vehicletreated rats (14.79±1.64%, P<0.01 vs. normal controls) (Fig. 2D).
The proliferation of MBP-specific T cells was significantly greater in vehicle-treated rats compared to in those treated with the I. okamurae ethyl acetate fraction (P<0.001) (Fig. 3). This reduction in T-cell proliferation ameliorated EAE-induced paralysis.
This study first demonstrates that I. okamurae delays the onset and ameliorates the severity of paralysis in EAE rats, possibly through the suppression of both T-cell proliferation and TCRαβ+ T-cells population, and reduction of pro-inflammatory mediators including TNF-α and COX-2. A similar anti-inflammatory activity of seaweeds including fucoidan, a sulfated polysaccharide from brown algae, has been reported previously in experimental pneumococcal meningitis [17] and perinatal hypoxic-ischemic encephalopathy [18]. Furthermore, fucoidan has been known to exert a therapeutic effect in rats with EAE by reducing the production of TNF-α and interleukin-10 [14].
As for the microglial activation in animal models of CNS diseases, the severity of neuro-inflammation can be scored by assessing the activation of microglial cells [19], which secrete various proinflammatory mediators [20]. EAE can also be caused by infiltration of autoimmune T cells and macrophages, with subsequent activation of microglia and astrocytes [1] and release of inflammatory cytokines, such as TNF-α and interleukins [1]. The Iba-1-positive area in CNS tissues reflects the severity of neuro-inflammation in a model of Theiler's murine encephalomyelitis virus-induced demyelination [21]. Thus it is postulated that I. okamurae treatment in EAE rats reduced microglial activation, possibly ameliorating EAE paralysis.
Proliferation of T cells in autoimmune disease is an important factor for the initiation of T cell mediated diseases [13]. In the present study, it was found that diphlorethohydroxycarmalol, an active compound of the ethyl acetate fraction of I. okamurae significantly suppressed T-cell proliferation in an antigen-dependent manner, as did in fucoidan treatment in EAE [14]. As for the T-cell phenotypes in rat EAE, TCRαβ+ T cells are prime cell types in rat EAE [3]. In this study, flow cytometric analysis revealed that reduced proportion of TCRαβ+ T cells in I. okamurae treated EAE rats would be associated with delayed onset of EAE paralysis. In the quantitative real time-PCR results, the ethyl-acetate fraction of I. okamurae inhibited the productions of TNF-α and COX-2, which are pro-inflammatory mediators, in EAE-induced rats. Pro-inflammatory cytokines including TNF-α has been known to be deeply associated with inflammatory cells in the acute phase [22]. These findings all suggest that the reduction of TCRαβ+ T-cells population and suppression of TNF-α and COX-2 in the I. okamurae treatment in EAE rats was partly associated with amelioration of rat EAE, as did in salicylate [23], a COX inhibitor, and phenidone [24], a dual inhibitor of COXs and lipoxygenase, treatment in EAE of rats.
Collectively, these results suggest that an I. okamurae ethanolic extract and/or its ethyl acetate fraction would be effective against MS through the suppression of T-cell proliferation as well as the reduction of pro-inflammatory mediators as far as rat EAE is concerned.
Figures and Tables
Fig. 1
Histopathological examination of the spinal cords of rats with experimental autoimmune encephalomyelitis (EAE) with or without Ishige okamurae treatment. There was no infiltration of inflammatory cells in the spinal cord of normal control rats (A, D). The spinal cords of vehicle-treated EAE rats contained many inflammatory cells (arrowheads) (B, E) in the parenchyma, whereas the number of inflammatory cells (arrowheads) (C, F) in the spinal cords of I. okamurae-treated rats (10 mg/kg body weight) was reduced. Hematoxylin and eosin staining. Arrowheads indicate inflammatory cells. Scale bars=200 µm (A–C) and 50 µm (D–F).
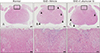
Fig. 2
Representative photographs of ionized calcium-binding adaptor molecule 1 (Iba-1) in the spinal cords of normal control (A), vehicle-treated (B), and Ishige okamurae-treated (C) experimental autoimmune encephalomyelitis (EAE) rats. (D) The Iba-1-positive area in the spinal cord of I. okamurae-treated EAE rats was significantly reduced compared with that of vehicle-treated EAE rats. *P<0.05. Insets indicate that higher-magnification photos. Immunostained for Iba-1 and counterstained with hematoxylin. Scale bars=200 µm (A–C), 50 µm (insets).
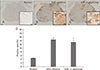
Fig. 3
Effect of Ishige okamurae on myelin basic protein-reactive T-cell responses (n=3). Data are means±SEM. CPM, count per minute; EAE, experimental autoimmune encephalomyelitis. ***P<0.001 vs. vehicle-treated rats.

Fig. 4
Ishige okamurae altered the population of mononuclear cells (MNCs) in spleen. (A) Splenic MNCs were isolated on day 10 postimmunization from rats treated with either vehicle or I. okamurae and analyzed by flow cytometry for TCRαβ+ T cells. The dot plot represents one of three separate experiments of similar observation. (B) Mean number of cell populations from splenic MNCs are plotted (n=3 each per group). Data are means±SEM. MNC, mononuclear cell. *P<0.05 vs. vehicle-treated rats.

Fig. 5
Quantitative real time polymerase chain reaction results of in the spinal cords of experimental autoimmune encephalomyelitis (EAE)-induced rats with or without Ishige okamurae at peak stage (n=3 per group). I. okamurae suppressed the pro-inflammatory mediator related genes in EAE-induced rats. The mRNA expression levels of tumor necrosis factor-α (TNF-α) (A) and cyclooxygenase-2 (COX-2) (B) in the spinal cords of I. okamurae-treated EAE rats was significantly decreased compared with that of vehicle-treated EAE rats. *P<0.05 vs. vehicle treated EAE rats.
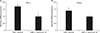
Table 1
Effect of Ishige okamurae ethanolic extract on the clinical symptoms of EAE in rats

Acknowledgements
This research was supported by the 2018 Scientic Promotion Program of Jeju National University.
References
1. Shin T, Ahn M, Matsumoto Y. Mechanism of experimental autoimmune encephalomyelitis in Lewis rats: recent insights from macrophages. Anat Cell Biol. 2012; 45:141–148.

2. Tanuma N, Shin T, Kogure K, Matsumoto Y. Differential role of TNF-alpha and IFN-gamma in the brain of rats with chronic relapsing autoimmune encephalomyelitis. J Neuroimmunol. 1999; 96:73–79.
3. Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998; 28:1681–1688.
4. Wang P, Xie K, Wang C, Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur Neurol. 2014; 72:249–254.

5. van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochim Biophys Acta. 2011; 1812:141–150.

6. Ljubisavljevic S, Stojanovic I, Pavlovic D, Sokolovic D, Stevanovic I. Aminoguanidine and N-acetyl-cysteine supress oxidative and nitrosative stress in EAE rat brains. Redox Rep. 2011; 16:166–172.
7. Lee IK, Kang JW. A check list of marine algae in Korea. Korean J Phycol. 1986; 1:311–325.
8. Kim MM, Rajapakse N, Kim SK. Anti-inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF-kappaB transcription factor in RAW 264.7 cells. Phytother Res. 2009; 23:628–634.
9. Heo SJ, Kim JP, Jung WK, Lee NH, Kang HS, Jun EM, Park SH, Kang SM, Lee YJ, Park PJ, Jeon YJ. Identification of chemical structure and free radical scavenging activity of diphlorethohydroxycarmalol isolated from a brown alga, Ishige okamurae. J Microbiol Biotechnol. 2008; 18:676–681.
10. Zou Y, Qian ZJ, Li Y, Kim MM, Lee SH, Kim SK. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agric Food Chem. 2008; 56:7001–7009.
11. Heo SJ, Cha SH, Kim KN, Lee SH, Ahn G, Kang DH, Oh C, Choi YU, Affan A, Kim D, Jeon YJ. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2-induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl Biochem Biotechnol. 2012; 166:1520–1532.
12. Ahn M, Moon C, Yang W, Ko EJ, Hyun JW, Joo HG, Jee Y, Lee NH, Park JW, Ko RK, Kim GO, Shin T. Diphlorethohydroxycarmalol, isolated from the brown algae Ishige okamurae, protects against radiation-induced cell damage in mice. Food Chem Toxicol. 2011; 49:864–870.
13. Shin T, Ahn M, Hyun JW, Kim SH, Moon C. Antioxidant marine algae phlorotannins and radioprotection: a review of experimental evidence. Acta Histochem. 2014; 116:669–674.

14. Kim H, Moon C, Park EJ, Jee Y, Ahn M, Wie MB, Shin T. Amelioration of experimental autoimmune encephalomyelitis in Lewis rats treated with fucoidan. Phytother Res. 2010; 24:399–403.

15. Ahn M, Yang W, Kim H, Jin JK, Moon C, Shin T. Immunohistochemical study of arginase-1 in the spinal cords of Lewis rats with experimental autoimmune encephalomyelitis. Brain Res. 2012; 1453:77–86.

16. Kim J, Choi Y, Ahn M, Jung K, Shin T. Olfactory dysfunction in autoimmune central nervous system neuroinflammation. Mol Neurobiol. 2018; 55:8499–8508.

17. Granert C, Raud J, Lindquist L. The polysaccharide fucoidin inhibits the antibiotic-induced inflammatory cascade in experimental pneumococcal meningitis. Clin Diagn Lab Immunol. 1998; 5:322–324.

18. Uhm CS, Kim KB, Lim JH, Pee DH, Kim YH, Kim H, Eun BL, Tockgo YC. Effective treatment with fucoidin for perinatal hypoxic-ischemic encephalopathy in rats. Neurosci Lett. 2003; 353:21–24.

19. Mecha M, Feliu A, Machín I, Cordero C, Carrillo-Salinas F, Mestre L, Hernández-Torres G, Ortega-Gutiérrez S, López-Rodríguez ML, de Castro F, Clemente D, Guaza C. 2-AG limits Theiler’s virus induced acute neuroinflammation by modulating microglia and promoting MDSCs. Glia. 2018; 66:1447–1463.

20. Li JJ, Liu SJ, Liu XY, Ling EA. Herbal compounds with special reference to gastrodin as potential therapeutic agents for microglia mediated neuroin fl ammation. Curr Med Chem. 2018; 02. 14. [Epub]. DOI: 10.2174/0929867325666180214123929.
21. Carrillo-Salinas FJ, Mestre L, Mecha M, Feliú A, Del Campo R, Villarrubia N, Espejo C, Montalbán X, Álvarez-Cermeño JC, Villar LM, Guaza C. Gut dysbiosis and neuroimmune responses to brain infection with Theiler's murine encephalomyelitis virus. Sci Rep. 2017; 7:44377.

22. Ahn M, Kang J, Lee Y, Riu K, Kim Y, Jee Y, Matsumoto Y, Shin T. Pertussis toxin-induced hyperacute autoimmune encephalomyelitis in Lewis rats is correlated with increased expression of inducible nitric oxide synthase and tumor necrosis factor alpha. Neurosci Lett. 2001; 308:41–44.

23. Moon C, Ahn M, Jee Y, Heo S, Kim S, Kim H, Sim KB, Koh CS, Shin YG, Shin T. Sodium salicylate-induced amelioration of experimental autoimmune encephalomyelitis in Lewis rats is associated with the suppression of inducible nitric oxide synthase and cyclooxygenases. Neurosci Lett. 2004; 356:123–126.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download