Abstract
Variations in the vascular anatomy of the carotid triangle have been reported in current scientific literature. The carotid arteries, being the major feeding arteries of the head and neck deserve special importance and protection from iatrogenic injury during radiological evaluations and surgical interventions. The present study was carried out over a period of 4 years from 2012–2016 to assess the variant anatomy of external carotid artery. The external carotid artery and its branches were dissected bilaterally in 40 formalin embalmed cadavers. The external carotid artery was traced from its origin to termination and variations in the branching pattern as well as the level of the carotid bifurcation were observed and analysed. A higher carotid bifurcation was observed in 25% cases. The linguofacial trunk was the commonest variation noted in the branching pattern seen in 20% cases. A single case of unilateral thyrolinguofacial trunk was also observed. The external carotid artery gave rise to accessory branches in 7.5% cases namely the superior laryngeal, accessory ascending pharyngeal and masseteric branches. A slender branch to the internal jugular vein was also observed in one case. These findings may provide further insight into the understanding of the vascular anatomy of the carotid triangle to the curious student, the discerning radiologist and the vigilant surgeon to avert complications and help improve overall treatment outcome.
The external carotid artery (ECA) and its branches serve as the major vascular channels of the head and neck region. The ECA arises in the carotid triangle from the common carotid artery (CCA) along with the internal carotid artery (ICA). The ECA is the main feeding vessel to the tissues of the head and neck region through its 8 branches, namely the superior thyroid artery (STA), ascending pharyngeal artery (APA), lingual artery (LA), facial artery (FA), occipital artery, posterior auricular artery (PAA), superficial temporal artery, and maxillary artery. In addition, the ECAs also play an important role in providing collateral blood supply to the brain through the many connections between the branches of the ECA and cranial branches of the ICA and vertebral arteries [1]. Injuries to the carotid arteries in cases of neck trauma is the cause of inaccessible exsanguinating haemorrhage, requiring emergency surgical intervention [23]. Pseudoaneurysms occurring consequent to blunt carotid injury commonly affect branches of the ECA than the main ECA itself [4]. The clinical significance of the ECA and its branches is further reinforced by their application in a wide range of radiological and surgical procedures such as intra-arterial infusion chemotherapy [56], carotid stenting and endarterectomy [78] as well as various head and neck surgeries [91011]. A comprehensive understanding of the normal as well as variant anatomy of the ECA is hence essential for the discerning radiologist as well as the judicious surgeon for successful completion of procedures and avoidance of vascular complications in the head and neck region.
The present prospective study was done over a period of 4 years from 2012 to 2016 after obtaining clearance from the institutional ethical clearance committee. 80 hemi-necks obtained from 40 formalin embalmed cadavers (32 male ad 8 female) were dissected and the external carotid arteries were traced from the origin to termination. Cadavers in which embalming had been done through CCA and those with injuries in the head and neck region were excluded from this study. The cadavers were aged between 50 and 80 years. Variations encountered in the origin and branching pattern of the ECA were documented with digital photography. The data was tabulated and the percentage of cadavers with variations in the branching pattern of ECA as well as variations in the level of the carotid bifurcation were computed and analysed.
The ECA took origin at the level of upper border of thyroid cartilage (TC) in 60/80 cases (75%). Higher level of origin was noted in the remaining 20 of 80 cases (25%). Higher levels of carotid bifurcation were further categorized keeping the TC as anatomical landmark (Table 1, Figs. 1,2,3). No lower levels of origin were noted in this study. The anteromedial position of the ECA relative to the ICA at the level of the carotid bifurcation was noted in all the cases.
The anterior branches of the ECA include the STA, LA, and FA. Out of the 80 hemi-necks studied, separate origins for the anterior branches of the ECA were observed in 63 cases (78.75%). In the remaining 17 cases, formation of common trunks was observed. The linguofacial trunk was the commonest observed variation with the thyrolinguofacial trunk (TLFT) occurring only in a single case (Fig. 4). The TLFT was found to arise 6 mm above the carotid bifurcation and after a short length of 3 mm, divided into the STA and a common linguofacial trunk (Fig. 4).
Out of the 16 linguofacial trunks noted, the trunks were bilaterally present in 10 of 80 cases (12.5%) and in the remaining 6 of 80 cases (7.5%), unilateral occurrence was observed. No thyrolingual trunks were observed. The findings are summarized in Table 2.
The APA is the smallest branch of the ECA and arises from its medial surface near the origin. Normal branching pattern of the APA was observed in 78/80 cases (97.5%). An unusually high origin of the APA was observed in 1 case (1.25%) where the APA arose 20 mm above the carotid bifurcation from the posterolateral aspect of the ECA, opposite to the LA (Fig. 5). In addition, double ascending pharyngeal arteries were observed arising from the posteromedial aspect of ECA in 1 case (1.25%) (Fig. 6).
No significant variations were observed in the branching pattern of the occipital and posterior auricular arteries.
The ECA terminated at the level of the neck of mandible by dividing into superficial temporal and maxillary arteries in all the cases. No variations were noted.
Accessory branches were found to arise from the ECA in 6 of 80 cases (7.5%) (Table 3).
The muscular branch to the masseter muscle was seen arising from the anterior aspect of the ECA just above the PAA in the parotid region (Fig. 7). A slender branch to the internal jugular vein was seen arising from the anteromedial aspect of ECA 3 mm above the STA origin (Fig. 8). Superior laryngeal artery (SLA) was found arising directly from ECA in 3.75% cases (Fig. 9).
Numerous variations in the external carotid vascular anatomy have been described in the available literature. The carotid bifurcation is an important anatomical and surgical landmark requiring special mention in the pathogenesis of carotid atheromatous disease and its consequent management by carotid stenting and endarterectomy [12]. A higher level carotid bifurcation may cause further distal extension of plaque making standard approaches inadequate for plaque removal and arteriotomy repair [13]. The level of the carotid bifurcation has been studied by various authors in the past. Higher levels of carotid bifurcation have been frequently reported in studies done by Lucev et al. [14], Ito et al. [15], Sanjeev et al. [16], Al-Rafiah et al. [17], and Mompeo and Bajo [18]. According to Al-Rafiah et al. [17] and Mompeo and Bajo [18], the commonest position of high carotid bifurcation was at the level of the hyoid bone in 25% and 36.85% cases respectively which correlates with our study. Lower levels of bifurcation have also been reported by the same authors with varying frequencies. Carotid bifurcations as low as intrathoracic bifurcations have also been reported in available literature [1920]. A low carotid bifurcation may be associated with Klippel-Feil anomaly [20] and may cause difficulties during surgeries like cervical discectomy [21]. With reference to previous similar studies (Table 4), it may be noted that a higher level bifurcation is more common and lower bifurcations are less frequent. This correlates well with the present study also where no low level bifurcations were found. At the same time, a high carotid bifurcation (CB) should caution surgeons regarding the close proximity of hypoglossal nerve and superior cervical ganglion as well as possible STA origin from the CB [2223]. The position of the CB depends on how low or high ECA originates from the third aortic arch [23].
It can thus be concluded that the level of the carotid bifurcation exhibits a great degree of anatomical variability and cautious ascertaining of its position is mandatory to avoid complications during angiographic and surgical interventions.
The occurrence of common trunks in the branching pattern of the ECA has frequently been described in the current literature. The linguofacial trunk, by far, appears to be the commonest variation with high incidence as per literature reports (Table 5). The linguofacial trunk was observed in 20% cases in the present study.
A unilateral TLFT was observed in a single case in our study (1.25%). This coincides with findings of previous studies where a TLFT is a relatively rare occurrence with the incidence reported to be 1.7% by Al-Rafiah et al. [17], 2.5% by Zumre et al. [24], and 0.3% by Vazquez et al. [28]. It is important to recognize these anatomical variations as the superior thyroid, facial and lingual arteries may be used as recipient vessels for microsurgical reconstruction in head and neck cancers [29].
Thyrolingual trunks have also been reported with a lesser incidence by Hayashi et al. [13] (1%), Ozgur et al. [25] (2.5%), and Acar et al. [27] (2%). However, no thyrolingual trunks were found in our study.
According to Baik et al. [30], common linguofacial trunks arising from the ECA may place the LA or FA in closer proximity to the tonsillar fossa, increasing the risk of iatrogenic vessel injury. The linguofacial trunk is also a common site of traumatic pseudoaneurysm after tonsillectomy [31].
Mata et al. [26] suggests that the embryology of the combined trunks would be in keeping with the angiogenesis theory, which suggests that confluence of the vessels and vessels with large diameter are more common in fetuses compared with adults as TLFTs are more common in fetuses and tend to disappear in adults. Iwai et al. [29] states that the TLFT represents the STA developing ectopically from the linguofacial trunk.
Knowledge of variations in the origin of the APA is vital for the exact identification of the neck vessels during surgery, to avoid a fatal mix-up with the ICA, as cited by Al-Rafiah et al. [17].
Hayashi et al. [13] observed the origin of APA from the ECA with relation to the origin of LA and found high origin above LA in 66% cases and below LA in 9% cases. We found a high origin of APA at the level of the LA in our study. Bergman et al. [32] states that the APA may be occasionally doubled or tripled. In the present study, an accessory APA with higher level of origin was observed in one case. Sanjeev et al. [16] observed accessory branches arising from the ECA in 13.5% cases as compared to 7.5% cases in the present study. When present, accessory branches of the ECA are often found to be the SLA as has been observed by the same authors previously [33]. The SLA arose from the ECA in 3.75% cases in the present study. This can be compared with the findings of Nayak et al. [34] where SLA arose from ECA in 5.4% cases and our previous study where SLA was found arising from ECA in 5% of the cases [33]. An accessory muscular branch supplying the masseter was seen in one case in this study. Masseteric branches from ECA have been observed by Marinho et al. [35] and Ariji et al. [36] in imaging studies. In addition, a slender branch to the internal jugular vein was seen arising from the anteromedial aspect of the ECA in the present study in one case. To the best of our knowledge, there are no previous reports of such a branch in the available literature. Standard textbooks [3738] describe the tunica adventitia of large arteries and veins as being supplied by their own vasa vasora which are derived from adjacent arteries.
The branches of the ECA are the key landmarks for adequate exposure and appropriate placement of cross-clamps to carry out successful removal of plaque and minimize postoperative complications in a bloodless surgical field [13]. Caution must be paid with ligature of blood vessels of the carotid triangle, because if these blood vessels are not distinguished, this may have catastrophic consequences in cerebral circulation or it can cause bleeding in the region of the ECA [39].
The branching pattern of the ECA in the neck shows considerable amount of variability and a clear anatomical understanding of the angioarchitecture will help to improve overall procedure outcome and prevent fatal complications. Prior angiographic assessment to ascertain the level of carotid bifurcation as well as the branching pattern of the carotid arterial system may prove valuable to avoid injury to vital structures such as hypoglossal nerve and minimise troublesome haemorrhage during surgical exploration of the head and neck region.
References
1. Reid DB, Irshad K, Miller S, Reid AW, Reid W, Diethrich EB. Endovascular significance of the external carotid artery in the treatment of cerebrovascular insufficiency. J Endovasc Ther. 2004; 11:727–733. PMID: 15615564.
2. Heltzel S, Jelinek L, Jaynes D. Variation in the caudal branches of the external carotid artery: comparison of sex and side. Med Res Arch. 2015; (1):1–10.
3. Mangla S, Sclafani SJ. External carotid arterial injury. Injury. 2008; 39:1249–1256. PMID: 18838134.
4. Nicoucar K, Popova N, Becker M, Dulguerov P. Pseudoaneurysm of the external carotid artery after a blunt facial trauma. J Trauma. 2008; 65:E24–E27. PMID: 17554220.
5. Ii N, Fuwa N, Toyomasu Y, Takada A, Nomura M, Kawamura T, Sakuma H, Nomoto Y. A novel external carotid arterial sheath system for intra-arterial infusion chemotherapy of head and neck cancer. Cardiovasc Intervent Radiol. 2017; 40:1099–1104. PMID: 28357576.
6. Tsurumaru D, Kuroiwa T, Yabuuchi H, Hirata H, Higaki Y, Tomita K. Efficacy of intra-arterial infusion chemotherapy for head and neck cancers using coaxial catheter technique: initial experience. Cardiovasc Intervent Radiol. 2007; 30:207–211. PMID: 17216381.
7. Xu DS, Abruzzo TA, Albuquerque FC, Dabus G, Eskandari MK, Guterman LR, Hage ZA, Hurley MC, Hanel RA, Levy EI, Nichols CW, Ringer AJ, Batjer HH, Bendok BR. External carotid artery stenting to treat patients with symptomatic ipsilateral internal carotid artery occlusion: a multicenter case series. Neurosurgery. 2010; 67:314–321. PMID: 20644416.
8. Al-Basheer M, Ferrar D, Nelson D, Vasudevan T. Outcome of the external carotid artery following carotid endarterectomy with added external carotid artery eversion endarterectomy. Ann Vasc Dis. 2011; 4:225–228. PMID: 23555457.
9. Won SY. Anatomical considerations of the superior thyroid artery: its origins, variations, and position relative to the hyoid bone and thyroid cartilage. Anat Cell Biol. 2016; 49:138–142. PMID: 27382516.
10. Banks ND, Hui-Chou HG, Tripathi S, Collins BJ, Stanwix MG, Nam AJ, Rodriguez ED. An anatomical study of external carotid artery vascular territories in face and midface flaps for transplantation. Plast Reconstr Surg. 2009; 123:1677–1687. PMID: 19483566.
11. Shang JB, Li YH, Chen Y, He XG, Zeng QL, Wang JY. Clinical application of transarterial embolization for massive hemorrhage in the nasopharyngeal and maxillofacial regions. Di Yi Jun Yi Da Xue Xue Bao. 2004; 24:210–212. PMID: 14965831.
12. Michalinos A, Chatzimarkos M, Arkadopoulos N, Safioleas M, Troupis T. Anatomical considerations on surgical anatomy of the carotid bifurcation. Anat Res Int. 2016; 2016:6907472. PMID: 27047690.
13. Hayashi N, Hori E, Ohtani Y, Ohtani O, Kuwayama N, Endo S. Surgical anatomy of the cervical carotid artery for carotid endarterectomy. Neurol Med Chir (Tokyo). 2005; 45:25–29. PMID: 15699617.
14. Lucev N, Bobinac D, Maric I, Drescik I. Variations of the great arteries in the carotid triangle. Otolaryngol Head Neck Surg. 2000; 122:590–591. PMID: 10740186.
15. Ito H, Mataga I, Kageyama I, Kobayashi K. Clinical anatomy in the neck region: the position of external and internal carotid arteries may be reversed. Okajimas Folia Anat Jpn. 2006; 82:157–167. PMID: 16526574.
16. Sanjeev IK, Anita H, Ashwini M, Mahesh U, Rairam GB. Branching pattern of external carotid artery in human cadavers. J Clin Diagn Res. 2010; 4:3128–3133.
17. Al-Rafiah A, EL-Haggagy AA, Aal IH, Zaki AI. Anatomical study of the carotid bifurcation and origin variations of the ascending pharyngeal and superior thyroid arteries. Folia Morphol (Warsz). 2011; 70:47–55. PMID: 21604253.
18. Mompeo B, Bajo E. Carotid bifurcation: clinical relevance. Eur J Anat. 2015; 19:37–42.
19. Gomez CK, Arnuk OJ. Intrathoracic bifurcation of the right common carotid artery. BMJ Case Rep. 2013; 2013:bcr2012007554.
20. Gailloud P, Murphy KJ, Rigamonti D. Bilateral thoracic bifurcation of the common carotid artery associated with Klippel-Feil anomaly. AJNR Am J Neuroradiol. 2000; 21:941–944. PMID: 10815673.
21. Gulsen S, Caner H, Altinors N. An anatomical variant: low-lying bifurcation of the common carotid artery, and its surgical implications in anterior cervical discectomy. J Korean Neurosurg Soc. 2009; 45:32–34. PMID: 19242568.
22. Lo A, Oehley M, Bartlett A, Adams D, Blyth P, Al-Ali S. Anatomical variations of the common carotid artery bifurcation. ANZ J Surg. 2006; 76:970–972. PMID: 17054544.
23. Kurkcuoglu A, Aytekin C, Oktem H, Pelin C. Morphological variation of carotid artery bifurcation level in digital angiography. Folia Morphol (Warsz). 2015; 74:206–211. PMID: 26050808.
24. Zümre O, Salbacak A, Ciçekcibaşi AE, Tuncer I, Seker M. Investigation of the bifurcation level of the common carotid artery and variations of the branches of the external carotid artery in human fetuses. Ann Anat. 2005; 187:361–369. PMID: 16163849.
25. Ozgur Z, Govsa F, Ozgur T. Assessment of origin characteristics of the front branches of the external carotid artery. J Craniofac Surg. 2008; 19:1159–1166. PMID: 18650752.
26. Mata JR, Mata FR, Souza MC, Nishijo H, Ferreira TA. Arrangement and prevalence of branches in the external carotid artery in humans. Ital J Anat Embryol. 2012; 117:65–74. PMID: 23420995.
27. Acar M, Salbacak A, Sakarya ME, Zararsiz I, Ulusoy M. The morphometrical analysis of the external carotid artery and its branches with multidetector computerized tomography angiography technique. Int J Morphol. 2013; 31:1407–1414.
28. Vazquez T, Cobiella R, Maranillo E, Valderrama FJ, McHanwell S, Parkin I, Sañudo JR. Anatomical variations of the superior thyroid and superior laryngeal arteries. Head Neck. 2009; 31:1078–1085. PMID: 19340860.
29. Iwai T, Izumi T, Inoue T, Fuwa N, Shibasaki M, Oguri S, Mitsudo K, Tohnai I. Thyrolinguofacial trunk arising from the carotid bifurcation determined by three-dimensional computed tomography angiography. Surg Radiol Anat. 2013; 35:75–78. PMID: 22855256.
30. Baik FM, Chang AA, Green DA, Pakbaz RS, Bergeron CM. Post-tonsillectomy lingual artery pseudoaneurysm. Laryngoscope. 2011; 121:S61. PMID: 21739628.
31. Manzato L, Trivelato FP, Alvarenga AY, Rezende MT, Ulhôa AC. Endovascular treatment of a linguofacial trunk pseudoaneurysm after tonsillectomy. Braz J Otorhinolaryngol. 2013; 79:524. PMID: 23929158.
32. Bergman RA, Afifi AK, Miyauchi R. Illustrated encyclopedia of human anatomic variation. Ascending pharyngeal artery [Internet]. Anatomy Atlases;c1995-2018. cited 2017 Apr 26. Available from: https://www.anatomyatlases.org/AnatomicVariants/Cardiovascular/Text/Arteries/PharyngealAscending.shtml.
33. Devadas D, Pillay M, Sukumaran TT. Variations in the origin of superior laryngeal artery. Anat Cell Biol. 2016; 49:254–258. PMID: 28127500.
34. Nayak SR, Krishnamurthy A, Prabhu LV, Potu BK, Bagoji IB, Jiji PJ, Chettiar GK. Variable origin of the superior laryngeal artery and its clinical significance. Al Ameen J Med Sci. 2011; 4:69–74.
35. Marinho LH, Shanahan DA, Langdon JD, Sinnatamby CS. The inferiorly based masseter muscle flap: anatomical basis for its use in head and neck reconstructive surgery. Int J Oral Maxillofac Surg. 1991; 20:100–105. PMID: 2051046.
36. Ariji Y, Kimura Y, Gotoh M, Sakuma S, Zhao YP, Ariji E. Blood flow in and around the masseter muscle: normal and pathologic features demonstrated by color Doppler sonography. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001; 91:472–482. PMID: 11312466.
37. Eroschenko VP. DiFiore's atlas of histology with functional correlations. 12th ed. New Delhi: Wolters Kluwer/Lippincott Williams & Wilkins Publishers;2014. p. 216–219.
38. Ross MH, Pawlina W. Histology: a text and atlas with correlated cell and molecular biology. 6th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins;2011. p. 408–414.
39. Delić J, Bajtarević A, Isaković E. Positional variations of the external and the internal carotid artery. Acta Med Salin. 2010; 39:86–89.
Fig. 1
High origin of left external carotid artery between upper border of thyroid cartilage and greater cornua of hyoid bone (2a). CCA, common carotid artery; ECA, external carotid artery; GC, greater cornua of hyoid bone; ICA, internal carotid artery; TC, thyroid cartilage.
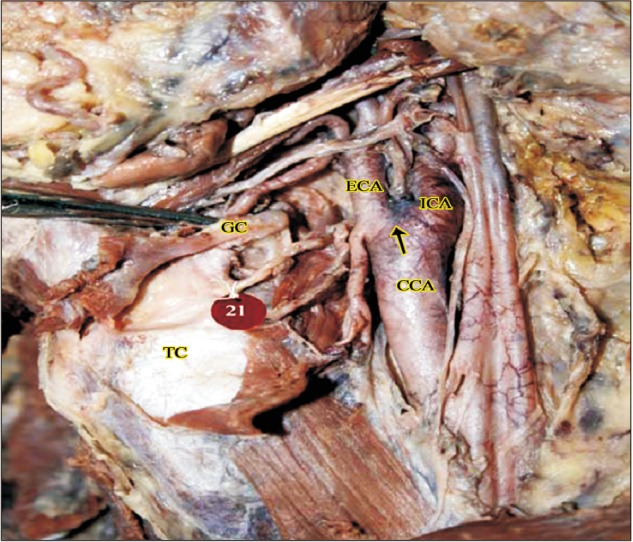
Fig. 2
Higher origin at the level of greater cornua of hyoid bone (right side, 2b). CCA, common carotid artery; DG, digastric; ECA, external carotid artery; GC, greater cornua of hyoid bone; HN, hypoglossal nerve; ICA, internal carotid artery; IJV, internal jugular vein; SH, stylohyoid; TC, thyroid cartilage.
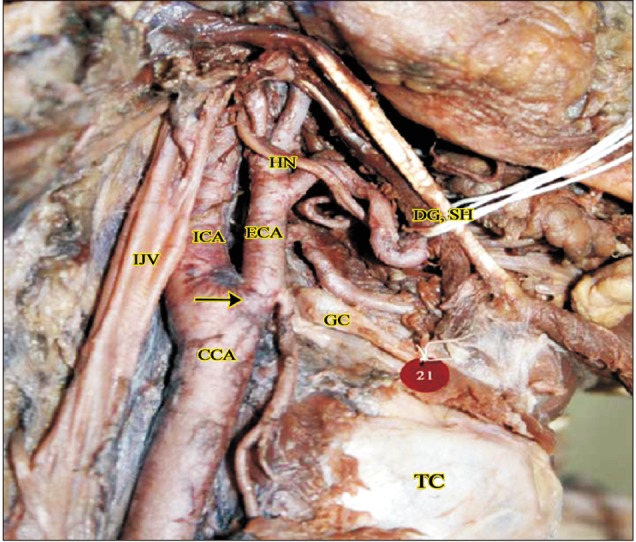
Fig. 3
Higher origin above the level of greater cornua of hyoid bone (right side, 2c). CCA, common carotid artery; ECA, external carotid artery; GC, greater cornua of hyoid bone; TC, thyroid cartilage.
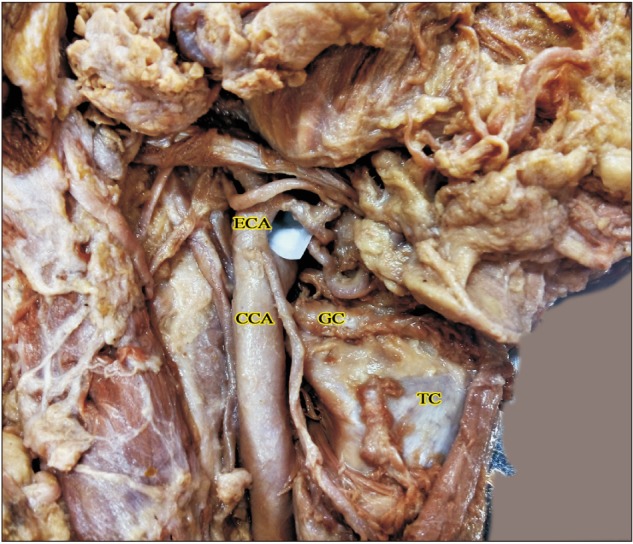
Fig. 4
Thyrolinguofacial trunk observed in a cadaver (right side). APA, ascending pharyngeal artery; CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; LA, lingual artery; LFT, linguofacial trunk; OA, occipital artery; SH, stylohyoid; SLA, superior laryngeal artery; SMG, submandibular gland; STA, superior thyroid artery; TLFT, thyrolinguofacial trunk.
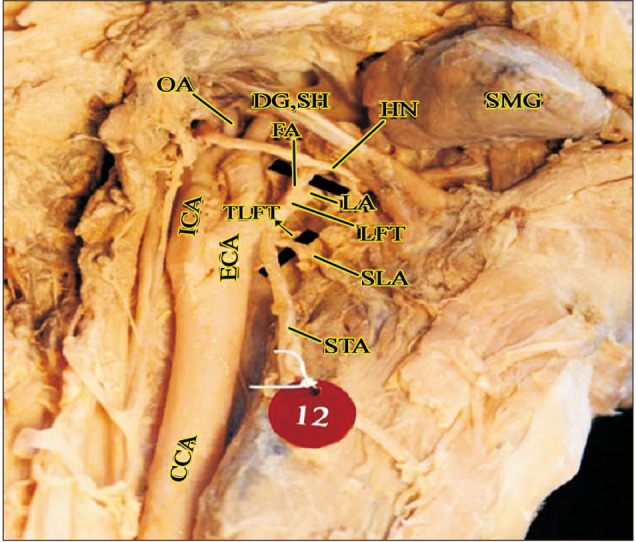
Fig. 5
High origin of ascending pharyngeal artery (left side). APA, ascending pharyngeal artery; CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; IJV, internal jugular vein; LA, lingual artery; OA, occipital artery; SH, stylohyoid; STA, superior thyroid artery.
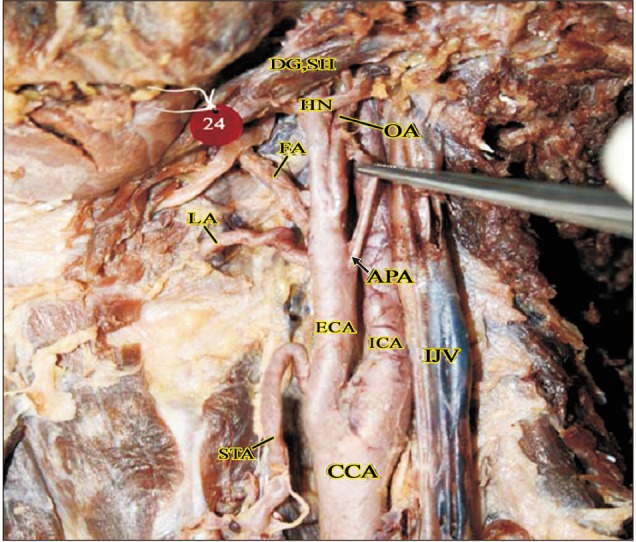
Fig. 6
Double ascending pharyngeal arteries in a cadaver (right side). APA, ascending pharyngeal artery; CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; LA, lingual artery; LFT, linguofacial trunk; OA, occipital artery; OH-S, omohyoid (superior belly); SH, stylohyoid; SLA, superior laryngeal artery; STA, superior thyroid artery.
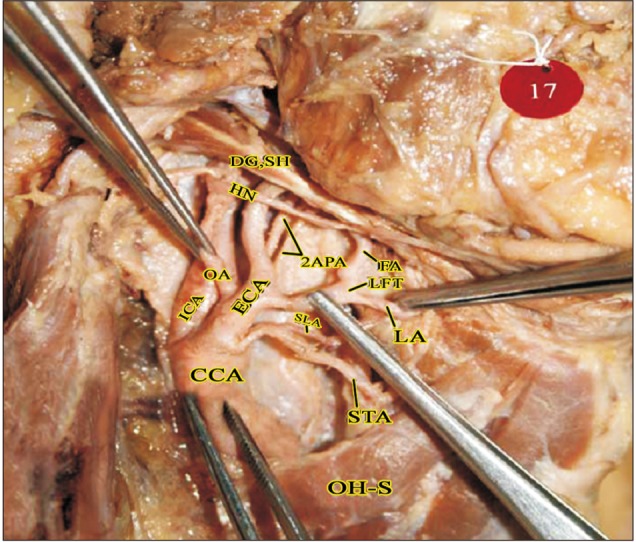
Fig. 7
Masseteric branch of ECA in the parotid region (right side). CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ICA, internal carotid artery; IJV, internal jugular vein; ILN, internal laryngeal nerve; LA, lingual artery; M, masseter; MB, muscular branch (to masseter); OA, occipital artery; OH-S, omohyoid (superior belly); PAA, posterior auricular artery; PG, parotid gland; SH, stylohyoid; SLA, superior laryngeal artery; STA, superior thyroid artery; VN, vagus nerve.
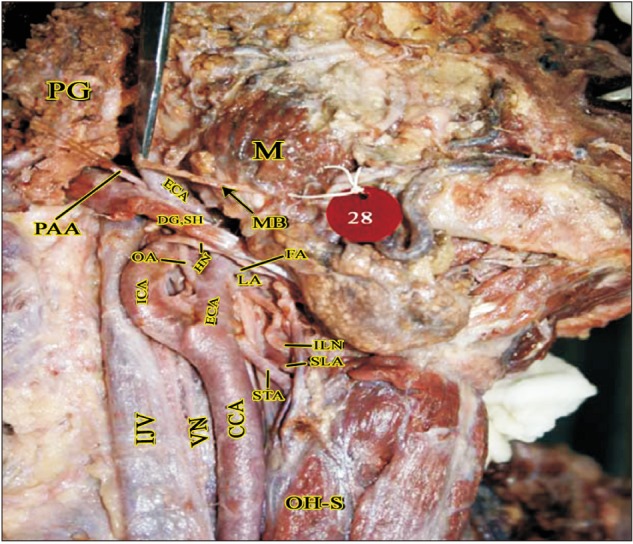
Fig. 8
Branch from ECA to Internal jugular vein (left side). Br.IJV, branch to internal jugular vein; CCA, common carotid artery; DG, digastric; ECA, external carotid artery; IJV, internal jugular vein; OH-S, omohyoid (superior belly); SH, stylohyoid; SMG, submandibular gland; STA, superior thyroid artery.
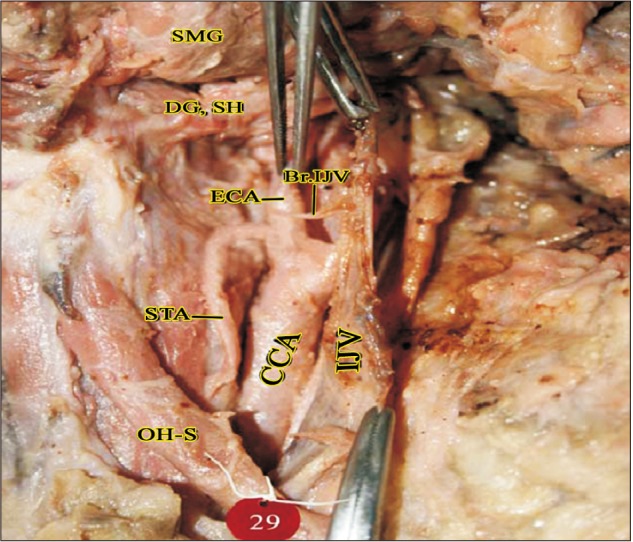
Fig. 9
SLA arising from ECA behind the TLFT (right side). CCA, common carotid artery; DG, digastric; ECA, external carotid artery; FA, facial artery; HN, hypoglossal nerve; ILN, internal laryngeal nerve; LA, lingual artery; SH, stylohyoid; SLA, superior laryngeal artery; STA, superior thyroid artery; TLFT, thyrolinguofacial trunk.
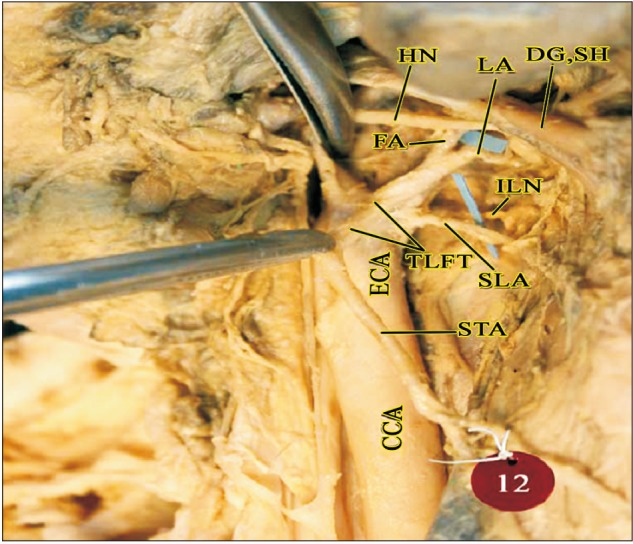
Table 1
Variations in the level of bifurcation of common carotid artery
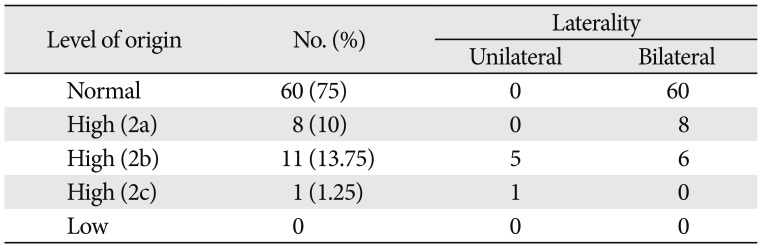
| Level of origin | No. (%) | Laterality | |
|---|---|---|---|
| Unilateral | Bilateral | ||
| Normal | 60 (75) | 0 | 60 |
| High (2a) | 8 (10) | 0 | 8 |
| High (2b) | 11 (13.75) | 5 | 6 |
| High (2c) | 1 (1.25) | 1 | 0 |
| Low | 0 | 0 | 0 |
Table 2
Variations in the anterior branches of external carotid artery
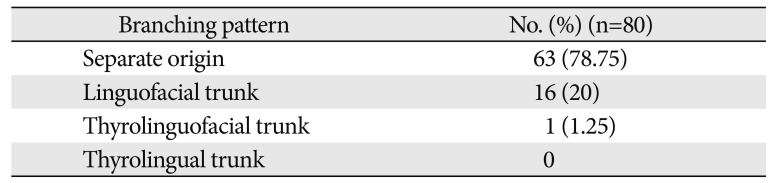
| Branching pattern | No. (%) (n=80) |
|---|---|
| Separate origin | 63 (78.75) |
| Linguofacial trunk | 16 (20) |
| Thyrolinguofacial trunk | 1 (1.25) |
| Thyrolingual trunk | 0 |
Table 3
Distribution of accessory branches of external carotid artery
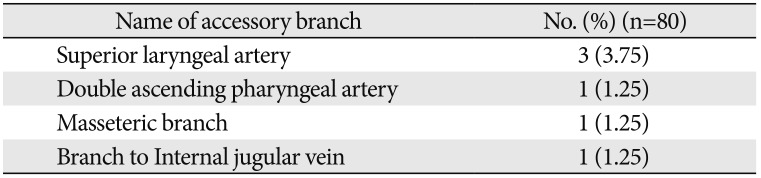
| Name of accessory branch | No. (%) (n=80) |
|---|---|
| Superior laryngeal artery | 3 (3.75) |
| Double ascending pharyngeal artery | 1 (1.25) |
| Masseteric branch | 1 (1.25) |
| Branch to Internal jugular vein | 1 (1.25) |
Table 4
Comparison of level of carotid bifurcation in the present study with previous studies
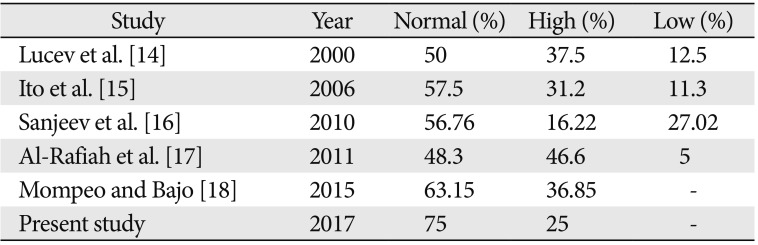
| Study | Year | Normal (%) | High (%) | Low (%) |
|---|---|---|---|---|
| Lucev et al. [14] | 2000 | 50 | 37.5 | 12.5 |
| Ito et al. [15] | 2006 | 57.5 | 31.2 | 11.3 |
| Sanjeev et al. [16] | 2010 | 56.76 | 16.22 | 27.02 |
| Al-Rafiah et al. [17] | 2011 | 48.3 | 46.6 | 5 |
| Mompeo and Bajo [18] | 2015 | 63.15 | 36.85 | - |
| Present study | 2017 | 75 | 25 | - |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download