1. Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959; 27:375–388.
2. Hung MJ, Hu P, Hung MY. Coronary artery spasm: review and update. Int J Med Sci. 2014; 11:1161–1171.

3. Slavich M, Patel RS. Coronary artery spasm: current knowledge and residual uncertainties. Int J Cardiol Heart Vasc. 2016; 10:47–53.

4. Shin DI, Baek SH, Her SH, Han SH, Ahn Y, Park KH, et al. The 24-month prognosis of patients with positive or intermediate results in the intracoronary ergonovine provocation test. JACC Cardiovasc Interv. 2015; 8:914–923.

5. Lanza GA, Sestito A, Sgueglia GA, Infusino F, Manolfi M, Crea F, et al. Current clinical features, diagnostic assessment and prognostic determinants of patients with variant angina. Int J Cardiol. 2007; 118:41–47.

6. Yasue H, Takizawa A, Nagao M, Nishida S, Horie M, Kubota J, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988; 78:1–9.

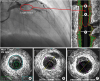
7. Yamagishi M, Miyatake K, Tamai J, Nakatani S, Koyama J, Nissen SE. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J Am Coll Cardiol. 1994; 23:352–357.

8. JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ J. 2014; 78:2779–2801.
9. Takagi Y, Yasuda S, Tsunoda R, Ogata Y, Seki A, Sumiyoshi T, et al. Clinical characteristics and long-term prognosis of vasospastic angina patients who survived out-of-hospital cardiac arrest: multicenter registry study of the Japanese Coronary Spasm Association. Circ Arrhythm Electrophysiol. 2011; 4:295–302.

10. Katayama T, Kubo N, Takagi Y, Funayama H, Ikeda N, Ishida T, et al. Relation of atherothrombosis burden and volume detected by intravascular ultrasound to angiographic no-reflow phenomenon during stent implantation in patients with acute myocardial infarction. Am J Cardiol. 2006; 97:301–304.

11. Sato H, Iida H, Tanaka A, Tanaka H, Shimodouzono S, Uchida E, et al. The decrease of plaque volume during percutaneous coronary intervention has a negative impact on coronary flow in acute myocardial infarction: a major role of percutaneous coronary intervention-induced embolization. J Am Coll Cardiol. 2004; 44:300–304.

12. Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011; 32:2059–2066.

13. Hong YJ, Ahn Y, Jeong MH. Role of intravascular ultrasound in patients with acute myocardial infarction. Korean Circ J. 2015; 45:259–265.

14. Hong YJ, Jeong MH, Choi YH, Ma EH, Ko JS, Lee MG, et al. Impact of baseline plaque components on plaque progression in nonintervened coronary segments in patients with angina pectoris on rosuvastatin 10 mg/day. Am J Cardiol. 2010; 106:1241–1247.

15. Stern S, Bayes de Luna A. Coronary artery spasm: a 2009 update. Circulation. 2009; 119:2531–2534.
16. Bertrand ME, LaBlanche JM, Tilmant PY, Thieuleux FA, Delforge MR, Carre AG, et al. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982; 65:1299–1306.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download