1. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007; 46:474–481. PMID:
17239480.

2. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010; 30:61–74. PMID:
20175034.

3. Sottani C, Poggi G, Quaretti P, Regazzi M, Montagna B, Quaquarini E, et al. Serum pharmacokinetics in patients treated with transarterial chemoembolization (TACE) using two types of epirubicin-loaded microspheres. Anticancer Res. 2012; 32:1769–1774. PMID:
22593459.
4. Jiaqi Y, Hori S, Minamitani K, Hashimoto T, Yoshimura H, Nomura N, et al. [A new embolic material: super absorbent polymer (SAP) microsphere and its embolic effects]. Nihon Igaku Hoshasen Gakkai Zasshi. 1996; 56:19–24. PMID:
8857094.
5. de Baere T, Plotkin S, Yu R, Sutter A, Wu Y, Cruise GM. An in vitro evaluation of four types of drug-eluting microspheres loaded with doxorubicin. J Vasc Interv Radiol. 2016; 27:1425–1431. PMID:
27402527.

6. Pereira PL, Plotkin S, Yu R, Sutter A, Wu Y, Sommer CM, et al. An in-vitro evaluation of three types of drug-eluting microspheres loaded with irinotecan. Anticancer Drugs. 2016; 27:873–878. PMID:
27416270.

7. Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, et al. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006; 17(2 Pt 1):335–342. PMID:
16517780.

8. Namur J, Wassef M, Millot JM, Lewis AL, Manfait M, Laurent A. Drug-eluting beads for liver embolization: concentration of doxorubicin in tissue and in beads in a pig model. J Vasc Interv Radiol. 2010; 21:259–267. PMID:
20123210.

9. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006; 12:2563–2567. PMID:
16638866.

10. Namur J, Citron SJ, Sellers MT, Dupuis MH, Wassef M, Manfait M, et al. Embolization of hepatocellular carcinoma with drug-eluting beads: doxorubicin tissue concentration and distribution in patient liver explants. J Hepatol. 2011; 55:1332–1338. PMID:
21703190.

11. Varela M, Sala M, Llovet JM, Bruix J. Treatment of hepatocellular carcinoma: is there an optimal strategy? Cancer Treat Rev. 2003; 29:99–104. PMID:
12670452.

12. van Malenstein H, Maleux G, Vandecaveye V, Heye S, Laleman W, van Pelt J, et al. A randomized phase II study of drugeluting beads versus transarterial chemoembolization for unresectable hepatocellular carcinoma. Onkologie. 2011; 34:368–376. PMID:
21734423.

13. Malagari K, Pomoni M, Moschouris H, Kelekis A, Charokopakis A, Bouma E, et al. Chemoembolization of hepatocellular carcinoma with HepaSphere 30-60 μm. Safety and efficacy study. Cardiovasc Intervent Radiol. 2014; 37:165–117. PMID:
24263774.
14. Emerich DF, Snodgrass P, Lafreniere D, Dean RL, Salzberg H, Marsh J, et al. Sustained release chemotherapeutic microspheres provide superior efficacy over systemic therapy and local bolus infusions. Pharm Res. 2002; 19:1052–1060. PMID:
12180539.
15. Lewis AL, Dreher MR, O'Byrne V, Grey D, Caine M, Dunn A, et al. DC BeadM1™: towards an optimal transcatheter hepatic tumour therapy. J Mater Sci Mater Med. 2016; 27:13. PMID:
26676859.

16. Lee S, Kim KM, Lee SJ, Lee KH, Lee DY, Kim MD, et al. Hepatic arterial damage after transarterial chemoembolization for the treatment of hepatocellular carcinoma: comparison of drug-eluting bead and conventional chemoembolization in a retrospective controlled study. Acta Radiol. 2017; 58:131–139. PMID:
27217418.

17. Kobayashi N, Ishii M, Ueno Y, Kisara N, Chida N, Iwasaki T, et al. Co-expression of Bcl-2 protein and vascular endothelial growth factor in hepatocellular carcinomas treated by chemoembolization. Liver. 1999; 19:25–31. PMID:
9928762.

18. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008; 49:523–529. PMID:
18568538.

19. Lee KH, Liapi E, Vossen JA, Buijs M, Ventura VP, Georgiades C, et al. Distribution of iron oxide-containing Embosphere particles after transcatheter arterial embolization in an animal model of liver cancer: evaluation with MR imaging and implication for therapy. J Vasc Interv Radiol. 2008; 19:1490–1496. PMID:
18755602.

20. Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017; 28:502–512. PMID:
27856136.

21. Lee JH, Won JH, Park SI, Won JY, Lee DY, Kang BC. Transcatheter arterial chemoembolization of hepatocellular carcinoma with hepatic arteriovenous shunt after temporary balloon occlusion of hepatic vein. J Vasc Interv Radiol. 2007; 18:377–382. PMID:
17377183.

22. European Association for Study of Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer. 2012; 48:599–641. PMID:
22424278.
23. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002; 35:519–524. PMID:
11870363.

24. Kim JH, Shim JH, Lee HC, Sung KB, Ko HK, Ko GY, et al. New intermediate-stage subclassification for patients with hepatocellular carcinoma treated with transarterial chemoembolization. Liver Int. 2017; 37:1861–1868. PMID:
28581250.

25. Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015; 21:10327–10335. PMID:
26420959.

26. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. ; PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010; 33:41–45. PMID:
19908093.
27. Hoóówko W, Wróblewski T, Wojtaszek M, Grąt M, Kobryń K, Ziarkiewicz-Wróblewska B, et al. Transarterial chemoembolization prior to liver transplantation in patients with hepatocellular carcinoma. Ann Transplant. 2015; 20:764–768. PMID:
26712800.

28. Yu CY, Ou HY, Weng CC, Huang TL, Chen TY, Leung-Chit L, et al. Drug-eluting bead transarterial chemoembolization as bridge therapy for hepatocellular carcinoma before living-donor liver transplantation. Transplant Proc. 2016; 48:1045–1048. PMID:
27320552.

29. Clavien PA, Lesurtel M, Bossuyt PM, Gores GJ, Langer B, Perrier A. OLT for HCC Consensus Group. Recommendations for liver transplantation for hepatocellular carcinoma: an international consensus conference report. Lancet Oncol. 2012; 13:e11–e22. PMID:
22047762.

30. Charnsangavej C. Chemoembolization of liver tumors. Semin Intervent Radiol. 1993; 10:150–160.

31. Sellers MT, Huggins S, Kegley K, Pollinger HS, Shrestha R, Johnson MW, et al. Multivariate analysis of prognostic factors for survival following doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2013; 24:647–654. PMID:
23384831.

32. Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, et al. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000; 88:50–57. PMID:
10618605.

33. Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. HPB (Oxford). 2010; 12:174–118. PMID:
20590884.

34. Fan J, Wu ZQ, Tang ZY, Zhou J, Qiu SJ, Ma ZC, et al. Multimodality treatment in hepatocellular carcinoma patients with tumor thrombi in portal vein. World J Gastroenterol. 2001; 7:28–32. PMID:
11819728.

35. Kim JH, Shim JH, Yoon HK, Ko HK, Kim JW, Gwon DI. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018; 38:1646–1654. PMID:
29436101.

36. Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, et al. Transcatheter treatment of hepatocellular carcinoma with doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012; 35:980–985. PMID:
22009576.

37. Kim HC. Role of C-arm cone-beam CT in chemoembolization for hepatocellular carcinoma. Korean J Radiol. 2015; 16:114–124. PMID:
25598679.

38. Tacher V, Radaelli A, Lin M, Geschwind JF. How I do it: cone-beam CT during transarterial chemoembolization for liver cancer. Radiology. 2015; 274:320–334. PMID:
25625741.

39. Choi JW, Kim HC, Lee JH, Yu SJ, Cho EJ, Kim MU, et al. Cone beam CT-guided chemoembolization of probable hepatocellular carcinomas smaller than 1 cm in patients at high risk of hepatocellular carcinoma. J Vasc Interv Radiol. 2017; 28:795–803.e1. PMID:
28302348.

40. Wang J, He XD, Zhang YC. Antibiotic prophylaxis in transarterial therapy of hepatocellular carcinoma: a meta-analysis. Can J Gastroenterol. 2012; 26:85–91. PMID:
22312607.

41. Lv WF, Lu D, He YS, Xiao JK, Zhou CZ, Cheng DL. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine (Baltimore). 2016; 95:e350.
42. Woo S, Chung JW, Hur S, Joo SM, Kim HC, Jae HJ, et al. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol. 2013; 200:1370–1377. PMID:
23701078.

43. Ji SK, Cho YK, Ahn YS, Kim MY, Park YO, Kim JK, et al. Multivariate analysis of the predictors of survival for patients with hepatocellular carcinoma undergoing transarterial chemoembolization: focusing on superselective chemoembolization. Korean J Radiol. 2008; 9:534–540. PMID:
19039270.
44. Lewis AL, Taylor RR, Hall B, Gonzalez MV, Willis SL, Stratford PW. Pharmacokinetic and safety study of doxorubicin-eluting beads in a porcine model of hepatic arterial embolization. J Vasc Interv Radiol. 2006; 17:1335–1343. PMID:
16923981.

45. Kim YW, Kwon JH, Nam SW, Jang JW, Jung HS, Shin YR, et al. Sustained multiple organ ischaemia after transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. Exp Ther Med. 2018; 15:1479–1483. PMID:
29434732.

46. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007; 5:1100–1108. PMID:
17627902.

47. Grosso M, Vignali C, Quaretti P, Nicolini A, Melchiorre F, Gallarato G, et al. Transarterial chemoembolization for hepatocellular carcinoma with drug-eluting microspheres: preliminary results from an Italian multicentre study. Cardiovasc Intervent Radiol. 2008; 31:1141–1149. PMID:
18696150.

48. Kalva SP, Iqbal SI, Yeddula K, Blaszkowsky LS, Akbar A, Wicky S, et al. Transarterial chemoembolization with doxorubicin-eluting microspheres for inoperable hepatocellular carcinoma. Gastrointest Cancer Res. 2011; 4:2–8. PMID:
21464864.
49. Manini MA, Sangiovanni A, Martinetti L, Viganò D, La Mura V, Aghemo A, et al. Transarterial chemoembolization with drug-eluting beads is effective for the maintenance of the Milan-in status in patients with a small hepatocellular carcinoma. Liver Transpl. 2015; 21:1259–1269. PMID:
26074360.

50. Sandow TA, Arndt SE, Albar AA, DeVun DA, Kirsch DS, Gimenez JM, et al. Assessment of response to transcatheter arterial chemoembolization with doxorubicin-eluting microspheres: tumor biology and hepatocellular carcinoma recurrence in a 5-year transplant cohort. Radiology. 2018; 286:1072–1083. PMID:
29206595.

51. Popovic P, Stabuc B, Jansa R, Garbajs M. Survival of patients with intermediate stage hepatocellular carcinoma treated with superselective transarterial chemoembolization using doxorubicin-loaded DC Bead under cone-beam computed tomography control. Radiol Oncol. 2016; 50:418–426. PMID:
27904450.

52. Prajapati HJ, Dhanasekaran R, El-Rayes BF, Kauh JS, Maithel SK, Chen Z, et al. Safety and efficacy of doxorubicin drug-eluting bead transarterial chemoembolization in patients with advanced hepatocellular carcinoma. J Vasc Interv Radiol. 2013; 24:307–315. PMID:
23375519.

53. Guiu B, Deschamps F, Aho S, Munck F, Dromain C, Boige V, et al. Liver/biliary injuries following chemoembolisation of endocrine tumours and hepatocellular carcinoma: lipiodol vs. drug-eluting beads. J Hepatol. 2012; 56:609–661. PMID:
22027582.

54. Malagari K, Pomoni M, Moschouris H, Bouma E, Koskinas J, Stefaniotou A, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Intervent Radiol. 2012; 35:1119–1128. PMID:
22614031.

55. Aliberti C, Carandina R, Lonardi S, Dadduzio V, Vitale A, Gringeri E, et al. Transarterial chemoembolization with small drug-eluting beads in patients with hepatocellular carcinoma: experience from a cohort of 421 patients at an Italian center. J Vasc Interv Radiol. 2017; 28:1495–1502. PMID:
28927662.

56. Burrel M, Reig M, Forner A, Barrufet M, de Lope CR, Tremosini S, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012; 56:1330–1133. PMID:
22314428.

57. Luz JH, Luz PM, Martin HS, Gouveia HR, Levigard RB, Nogueira FD, et al. DEB TACE for intermediate and advanced HCC - Initial experience in a Brazilian Cancer Center. Cancer Imaging. 2017; 17:5. PMID:
28166821.

58. Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014; 37:381–387. PMID:
23754191.

59. Song MJ, Chun HJ, Song DS, Kim HY, Yoo SH, Park CH, et al. Comparative study between doxorubicin-eluting beads and conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Hepatol. 2012; 57:1244–1250. PMID:
22824821.

60. Ferrer Puchol MD, la Parra C, Esteban E, Vaño M, Forment M, Vera A, et al. [Comparison of doxorubicin-eluting bead transarterial chemoembolization (DEB-TACE) with conventional transarterial chemoembolization (TACE) for the treatment of hepatocellular carcinoma]. Radiologia. 2011; 53:246–253. PMID:
21295802.

61. Kloeckner R, Weinmann A, Prinz F, Pinto dos Santos D, Ruckes C, Dueber C, et al. Conventional transarterial chemoembolization versus drug-eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015; 15:465. PMID:
26059447.

62. Kucukay F, Badem S, Karan A, Ozdemir M, Okten RS, Ozbulbul NI, et al. A single-center retrospective comparison of doxorubicin-loaded HepaSphere transarterial chemoembolization with conventional transarterial chemoembolization for patients with unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2015; 26:1622–1629. PMID:
26321015.

63. Arabi M, BenMousa A, Bzeizi K, Garad F, Ahmed I, Al-Otaibi M. Doxorubicin-loaded drug-eluting beads versus conventional transarterial chemoembolization for nonresectable hepatocellular carcinoma. Saudi J Gastroenterol. 2015; 21:175–180. PMID:
26021777.

64. Megías Vericat JE, García Marcos R, López Briz E, Gómez Muñoz F, Ramos Ruiz J, Martínez Rodrigo JJ, et al. Trans-arterial chemoembolization with doxorubicin-eluting particles versus conventional trans-arterial chemoembolization in unresectable hepatocellular carcinoma: a study of effectiveness, safety and costs. Radiologia. 2015; 57:496–504. PMID:
25857250.

65. Rahman FA, Naidu J, Ngiu CS, Yaakob Y, Mohamed Z, Othman H, et al. Conventional versus doxorubicin-eluting beads transarterial chemoembolization for unresectable hepatocellular carcinoma: a tertiary medical centre experience in Malaysia. Asian Pac J Cancer Prev. 2016; 17:4037–4041. PMID:
27644658.
66. Baur J, Ritter CO, Germer CT, Klein I, Kickuth R, Steger U. Transarterial chemoembolization with drug-eluting beads versus conventional transarterial chemoembolization in locally advanced hepatocellular carcinoma. Hepat Med. 2016; 8:69–74. PMID:
27382341.

67. Massani M, Stecca T, Ruffolo C, Bassi N. Should we routinely use DEBTACE for unresectable HCC? cTACE versus DEBTACE: a single-center survival analysis. Updates Surg. 2017; 69:67–73. PMID:
28097502.

68. Lee YK, Jung KS, Kim DY, Choi JY, Kim BK, Kim SU, et al. Conventional versus drug-eluting beads chemoembolization for hepatocellular carcinoma: emphasis on the impact of tumor size. J Gastroenterol Hepatol. 2017; 32:487–496. PMID:
27503585.

69. Liu YS, Ou MC, Tsai YS, Lin XZ, Wang CK, Tsai HM, et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015; 16:125–132. PMID:
25598680.

70. Gao S, Yang Z, Zheng Z, Yao J, Deng M, Xie H, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013; 60:813–820. PMID:
23282741.
71. Huang K, Zhou Q, Wang R, Cheng D, Ma Y. Doxorubicin-eluting beads versus conventional transarterial chemoembolization for the treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2014; 29:920–925. PMID:
24224722.

72. Xie ZB, Wang XB, Peng YC, Zhu SL, Ma L, Xiang BD, et al. Systematic review comparing the safety and efficacy of conventional and drug-eluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015; 45:190–200. PMID:
25388603.

73. Chen P, Yuan P, Chen B, Sun J, Shen H, Qian Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017; 41:75–85. PMID:
27350573.

74. Facciorusso A, Di Maso M, Muscatiello N. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Dig Liver Dis. 2016; 48:571–577. PMID:
26965785.

75. Gomes AS, Monteleone PA, Sayre JW, Finn RS, Sadeghi S, Tong MJ, et al. Comparison of triple-drug transcatheter arterial chemoembolization (TACE) with single-drug TACE using doxorubicin-eluting beads: long-term survival in 313 patients. AJR Am J Roentgenol. 2017; 209:722–732. PMID:
28705059.

76. Dinca H, Pelage JP, Baylatry MT, Ghegediban SH, Pascale F, Manfait M, et al. Why do small size doxorubicin-eluting microspheres induce more tissue necrosis than larger ones? A comparative study in healthy pig liver (oral communication 2206-2). In : CIRSE Annual meeting; 2012 September, 15-19; Lisbon, Portugal.
77. Seki A, Hori S, Kobayashi K, Narumiya S. Transcatheter arterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single-center experience. Cardiovasc Intervent Radiol. 2011; 34:557–565. PMID:
20821211.

78. Dreher MR, Sharma KV, Woods DL, Reddy G, Tang Y, Pritchard WF, et al. Radiopaque drug-eluting beads for transcatheter embolotherapy: experimental study of drug penetration and coverage in swine. J Vasc Interv Radiol. 2012; 23:257–264.e4. PMID:
22178039.

79. Spreafico C, Cascella T, Facciorusso A, Sposito C, Rodolfo L, Morosi C, et al. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Intervent Radiol. 2015; 38:129–134. PMID:
24870698.

80. Pua U. “Deploy and retrieve” technique using detachable micro-coil for temporary occlusion during drug-eluting beads chemoembolization. Cardiovasc Intervent Radiol. 2015; 38:1359–1362. PMID:
25672285.

81. Todoroki W, Hirakawa M, Nagao E, Soeda H, Tsuruta S, Honda H. Transarterial chemoembolization for hepatocellular carcinoma using a new double-lumen microballoon catheter with a side hole. J Vasc Interv Radiol. 2014; 25:1485–1486. PMID:
25150909.

82. Levy EB, Krishnasamy VP, Lewis AL, Willis S, Macfarlane C, Anderson V, et al. First human experience with directly image-able iodinated embolization microbeads. Cardiovasc Intervent Radiol. 2016; 39:1177–1186. PMID:
27206503.

83. Aliberti C, Carandina R, Sarti D, Pizzirani E, Ramondo G, Cillo U, et al. Transarterial chemoembolization with DC Bead LUMI™ radiopaque beads for primary liver cancer treatment: preliminary experience. Future Oncol. 2017; 13:2243–2252. PMID:
29063780.

84. Suk Oh J, Jong Chun H, Gil Choi B, Giu Lee H. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma: usefulness of contrast saturation features on cone-beam computed tomography imaging for predicting short-term tumor response. J Vasc Interv Radiol. 2013; 24:483–489. PMID:
23452553.

85. Golowa YS, Cynamon J, Reinus JF, Kinkhabwala M, Abrams M, Jagust M, et al. Value of noncontrast CT immediately after transarterial chemoembolization of hepatocellular carcinoma with drug-eluting beads. J Vasc Interv Radiol. 2012; 23:1031–1035. PMID:
22739645.


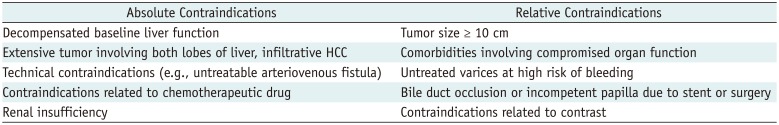
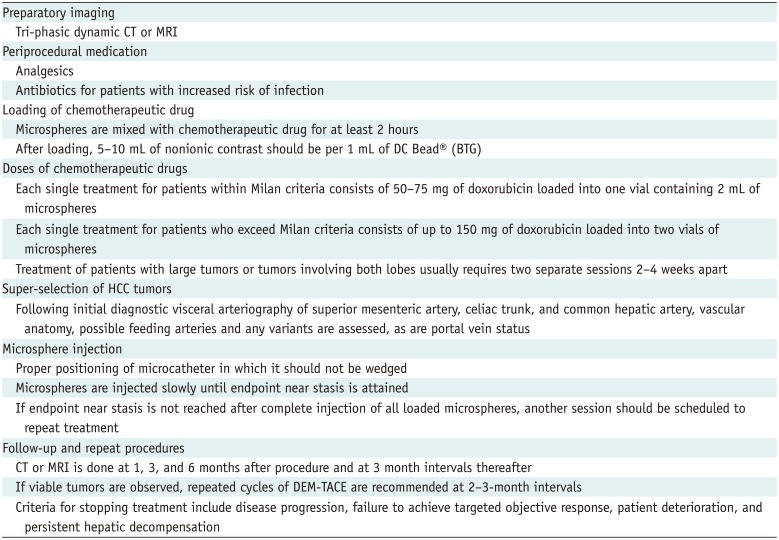
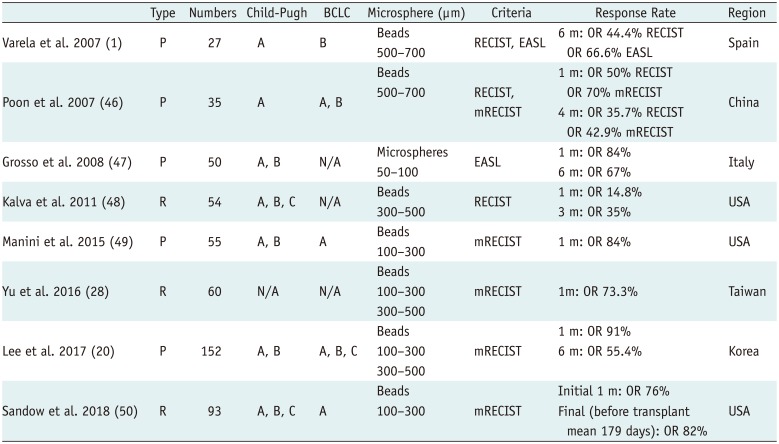




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download