Abstract
Purpose
Methods
Results
ACKNOWLEDGMENTS
References
Figure 1
Schematic figure of contrast-enhanced digital mammography, according to the time line; the image was provided courtesy of Hologic.
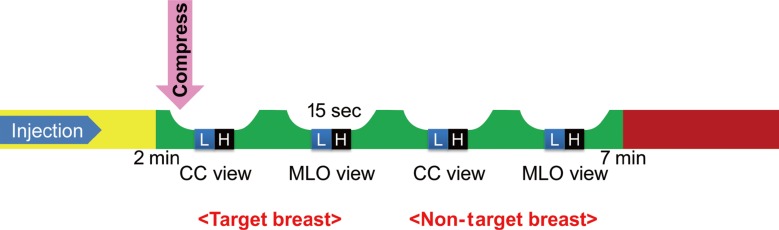
Figure 2
Correlation of the final assessment of contrast-enhanced digital mammography (CEDM) and contrast-enhanced magnetic resonance imaging (CEMRI) with histopathologic results.
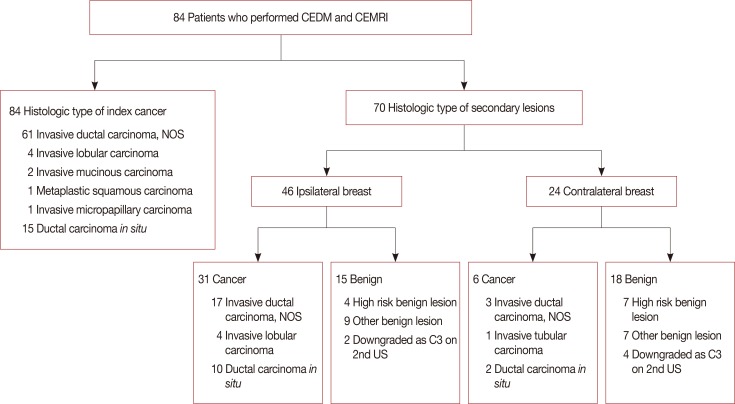
Figure 3
An asymptomatic 49-year-old woman confirmed with ductal carcinoma in situ (DCIS) on her left breast with previous history of breast-conserving surgery due to invasive ductal carcinoma on ipsilateral side of breast. (A) On contrast-enhanced magnetic resonance imaging (CEMRI), symmetric severe background parenchymal enhancement was noted. Axial maximal intensity projection image showed index cancer with regional non-mass enhancement (square) around previous operation site on her left breast and unexpected enhancing mass (arrow) in contralateral right breast. (B) On contrast-enhanced digital mammography, there was a regional non-mass enhancement correlated with index cancer (square) in left lower outer quadrant. And contralateral irregular enhancing mass (arrow) was also seen in right upper medial breast, identical to CEMRI. She underwent bilateral mastectomy, and the result was DCIS for left breast, and invasive tubular carcinoma for contralateral right breast (true positive).
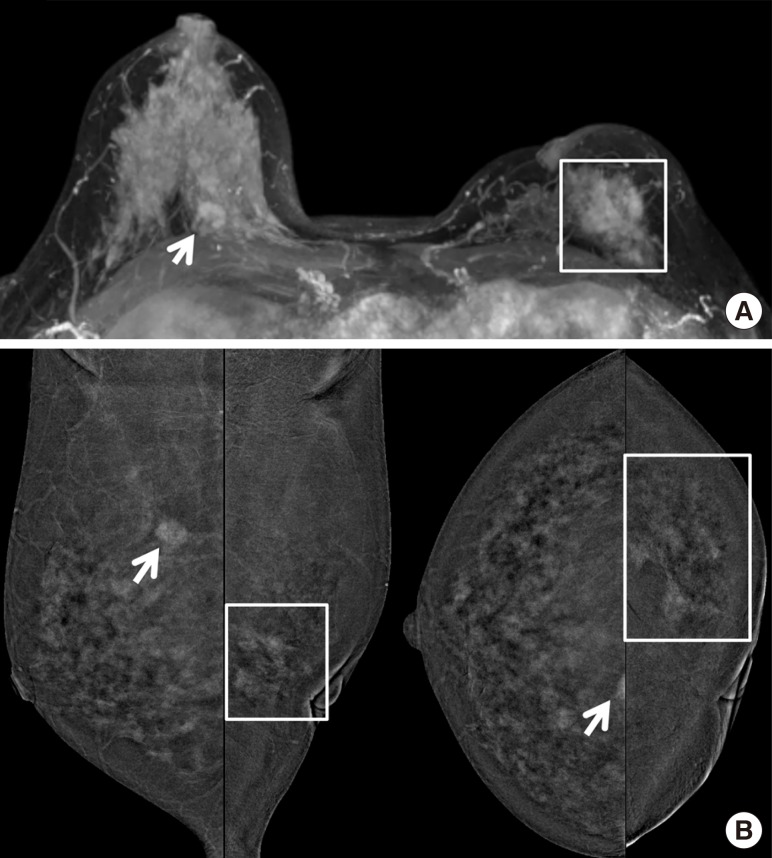
Figure 4
A 38-year-old woman with proven invasive ductal carcinoma in her right breast. (A) She underwent contrast-enhanced magnetic resonance imaging, and axial maximal intensity projection image showed index cancer (arrows) with unexpected subareolar enhancing small mass (arrowheads) in right breast. (B) On contrast-enhanced digital mammography, the index cancer (arrow) was well visualized with another subareolar enhancing mass (arrowhead) in right breast. She underwent breast-conserving surgery for right breast, and the result was single invasive ductal carcinoma. A subareolar small enhancing mass was false positive lesion which confirmed as fibroadenomatoid lesion.
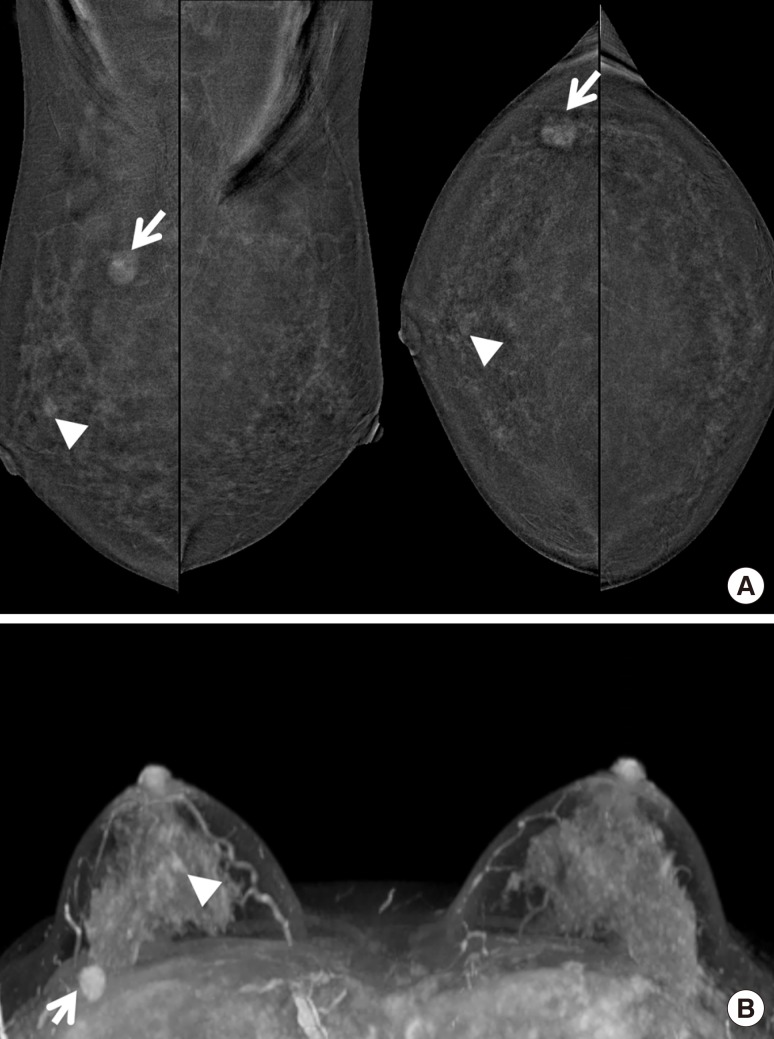
Figure 5
The effect of contrast-enhanced digital mammography (CEDM) and contrast-enhanced magnetic resonance imaging (CEMRI) on the surgical management of breast cancer patients.
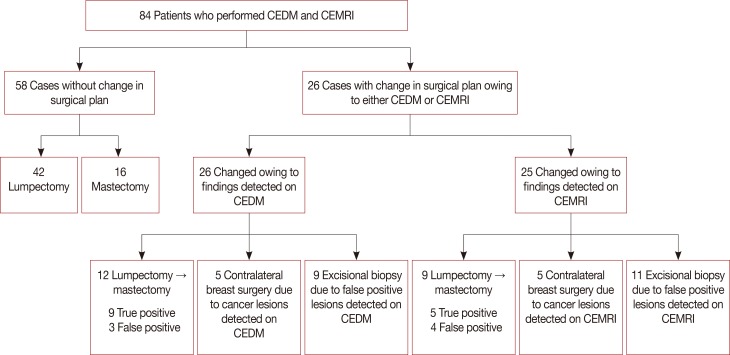
Table 1
The correlation of CEDM and CEMRI final assessments with histopathologic results
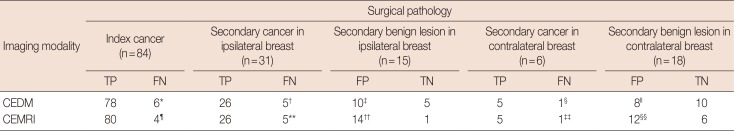
CEDM=contrast-enhanced digital mammography; CEMRI=contrast-enhanced magnetic resonance imaging; TP=true positive; FN=false negative; FP=false positive; TN=true negative.
*2: invasive ductal carcinoma (IDC), 4: ductal carcinoma in situ (DCIS); †1: IDC, 4: DCIS; ‡2: high-risk benign lesions, 7: other benign lesions, 1: no suspicious finding at 2nd ultrasonography (US); §1: DCIS; ∥3: high-risk benign lesions, 4: other benign lesions, 1: no suspicious finding at 2nd US; ¶1: IDC, 1: invasive lobular carcinoma (ILC), 2: DCIS; **1: IDC, 1: ILC, 3: DCIS; ††3: high-risk benign lesions, 9: other benign lesions, 2: no suspicious finding at 2nd US; ‡‡1: IDC; §§5: high-risk benign lesions, 4: other benign lesions, 3: no suspicious finding at 2nd US.
Table 2
Comparison of the diagnostic performance of CEDM and CEMRI for detecting secondary cancers in ipsilateral and contralateral breast
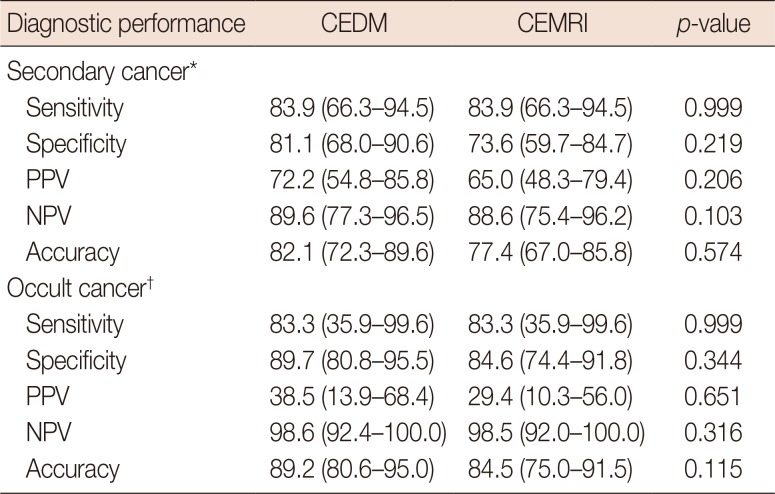
Data in parentheses are 95% confidence intervals.
CEDM=contrast-enhanced digital mammography; CEMRI=contrast-enhanced magnetic resonance imaging; PPV =positive predictive value; NPV=negative predictive value.
*Secondary cancer (multifocality and multicentricity) in the ipsilateral breast (n=31); †Occult cancer in the contralateral breast (n=6).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download