Abstract
Purpose
The definition of nodal pathologic complete response (pCR) after a neoadjuvant chemotherapy (NAC) just included the evaluation of axillary lymph node (ALN) without internal mammary lymph node. This study aimed to evaluate the feasibility of internal mammary-sentinel lymph node biopsy (IM-SLNB) in patients with breast cancer who underwent NAC.
Methods
From November 2011 to 2017, 179 patients with primary breast cancer who underwent operation after NAC were included in this study. All patients received radiotracer injection with modified injection technology. IM-SLNB would be performed on patients with internal mammary sentinel lymph node (IMSLN) visualization.
Results
Among the 158 patients with cN+ disease, the rate of nodal pCR was 36.1% (57/158). Among the 179 patients, the visualization rate of IMSLN was 31.8% (57/179) and was 12.3% (7/57) and 87.7% (50/57) among those with cN0 and cN+ disease, respectively. Furthermore, the detection rate of IMSLN was 31.3% (56/179). The success rate of IM-SLNB was 98.2% (56/57). The IMSLN metastasis rate was 7.1% (4/56), and all of them were accompanied by ALN metastasis. The number of positive ALNs in patients with IMSLN metastasis was 3, 6, 8, and 9. The pathology nodal stage had been changed from pN1/pN2 to pN3b. The pathology stage had been changed from IIA/IIIA to IIIC.
Neoadjuvant chemotherapy (NAC) is the standard treatment for patients with locally advanced as well as some stage II to III human epidermal growth factor receptor 2 (HER2) positive (HER2+) and triple-negative (TN) breast cancer. It could improve the rate of breast-conserving surgery (BCS) and offer an opportunity to evaluate the efficacy of chemotherapy on primary tumor in vivo [1]. The pathologic complete response (pCR) has been shown to be associated with improved survival outcomes, and nodal pCR is defined as no tumor cell remaining in the axillary lymph node (ALN) [2]. This definition of nodal pCR only focuses on the pathological state of ALN that overlooks the internal mammary lymph node (IMLN), which is one of the most important metastatic pathways of breast cancer [3]. IMLN usually has deep anatomical position and small diameter (<0.5 cm), the accuracy of imaging examination does not reflect the metastasis of IMLN. Furthermore, fine needle aspiration biopsy may not be able to reach the proper position and get false-negative results. With the development of axillary-sentinel lymph node biopsy (A-SLNB), internal mammary-sentinel lymph node biopsy (IM-SLNB) is expected to be a minimally invasive diagnostic technology for evaluating the metastasis of IMLN [4]. By assessing the visualization and metastatic rates of internal mammary sentinel lymph node (IMSLN) after NAC, our study aimed to evaluate the feasibility of IM-SLNB after NAC in patients with breast cancer.
A total of 179 female patients who had a histology-confirmed initial clinical staging of T1–4N0–3M0 in invasive breast cancer treated at Shandong Cancer Hospital Breast Cancer Center were enrolled in this study between November 2011 and 2017. The study was approved by the Shandong Cancer Hospital Affiliated to Shandong University Ethics Committee (No. SDTHEC20110324). Informed consent was obtained from all patients, and all procedures were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration. Patients diagnosed with distant metastatic disease prior to surgery or who did not have surgery at our center were excluded. Clinicopathologic and treatment data were collected from the patients' medical record. Before NAC, all patients underwent a series of evaluation by a multidisciplinary team. Suspicious positive ALNs were accessed using fine needle aspiration prior to the initiation of NAC. One percent expression rate was used as the standard to define positive hormone receptors (HRs). HER2 receptor was considered positive with immunohistochemical staining of 3+ or fluorescence in situ hybridization that was amplified. All patients received full course of chemotherapy regimens before surgery. The primary end-point was nodal pCR means no residual carcinoma in ALNs. Total pCR was defined as no residual invasive carcinoma in both breast and ALNs. Patients who were clinically node negative (n=21) at presentation were excluded from the nodal pCR end-point calculation.
All patients received dual tracer injection of radiolabeled colloid and blue dye. Radiotracer was injected with modified injection technique: sulfur colloid was labeled with 99mTc after filtering through a Millipore filter with a pore size of 220 nm (Beijing Atomic Galactic Jinan Drug Center, Beijing, China), with the guidance of ultrasound, and 18–37 MBq of 99mTc-labeled sulfur colloid (1.0–1.2 mL volume) was injected into the mammary gland at 6 and 12 o'clock of the areola surrounding area 3–18 hours before surgery [56]. Preoperative SPECT/CT lymphoscintigraphy (Philips Electronic N.V, Beijing, China) was performed before surgery. Blue dye (methylene blue) (2–4 mL) was injected subcutaneously around the tumor 10 minutes before surgery.
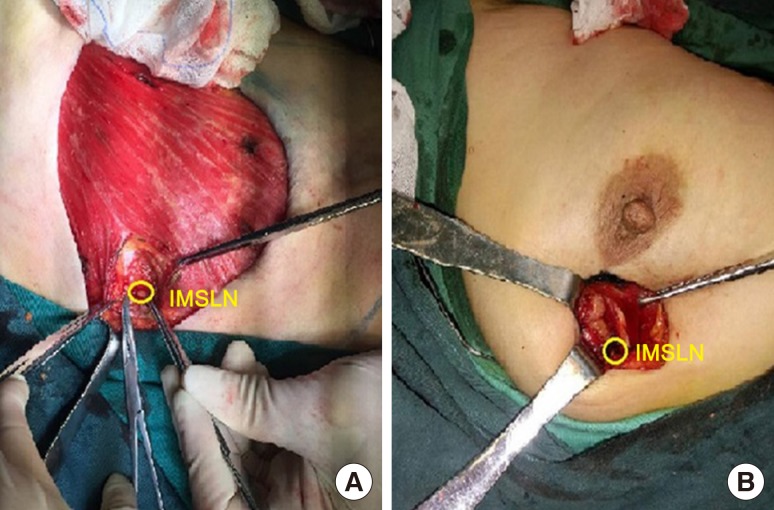
Finishing the surgery of axillary and breast, IM-SLNB was performed on patients with visualization of IMSLN, which was detected by preoperative lymphoscintigraphy and/or gamma probe technique. From the sternal border in a lateral direction 3.0 cm to 4.0 cm, the pectoral major muscle fibers were separated to expose the posterior intercostal space. The external and internal intercostal muscles were divided from the sternal border. In this procedure, particular care must be taken to avoid injury to the inferior parietal pleura and the internal mammary vessels. Then, IMSLNs were removed through the intercostal space with the help of the gamma probe technique. In the surgery, we also need to record nuclide count and location of IMSLN. In patients who received mastectomy, IM-SLNB was performed using the mastectomy incision. In patients who received BCS, if the incision was improper, IM-SLNB should be performed with another incision (Figure 1). Lymph nodes who found to have any tumor in itself, including micrometastases and isolated tumor cells, were defined as positive.
All statistical data were analyzed with SPSS version 22.0 software (IBM Corp., Armonk, USA). Independent-sample t-test was used for continuous variables, and Pearson chi-square test or Fisher exact test was used for categorical variables. A p<0.05 was considered statistically significant.
From November 2011 to 2017, 179 patients received full course of chemotherapeutic regimens followed by surgery in our cancer center. The median age of these patients was 49 years (range, 25–70 years). In this study, 49.7% (89/179) of patients had HR+/HER2− disease, 33.0% (59/179) had HER2+ disease, and the remaining 17.3% (31/179) had TN disease. The most common chemotherapeutic regimens that patients received included pharmorubicin, cyclophosphamide, and paclitaxel. HER2+ disease was identified in 59 patients, 49.2% (29/59) of them received anti-HER2-targeted therapy (trastuzumab) before surgery. In terms of breast surgery of the selected, 81.6% (146/179) of patients underwent mastectomy, and the other 18.4% (33/179) of patients received BCS.
In general, 17.9% (32/179) of patients achieved total pCR and was significantly associated with tumor subtype (p<0.001). About 5.6%, 25.8%, 48.3%, and 16.7% in patients with HR+/HER2−, TN, HER2+ with, and HER2+ without targeted therapy.
Among the 158 cN+ patients, nodal pCR rate was 36.1% (57/158). Our data also indicated that the nodal pCR has nothing to do with tumor burden in ALNs but was related to tumor subtype and was also significantly higher in patients with TN (44.5%) and HER2+ (with and without targeted therapy was 76.0% and 34.6%, respectively) disease than in those with HR+/HER2− disease (21.3%, p<0.001). Our data also indicated that the nodal pCR has nothing to do with tumor burden in ALNs but related to tumor subtype. The association between clinical nodal stage and nodal pCR was no longer significant: cN1 (42.7%), cN2 (35.2%), and cN3 (20.7%, p=0.110).
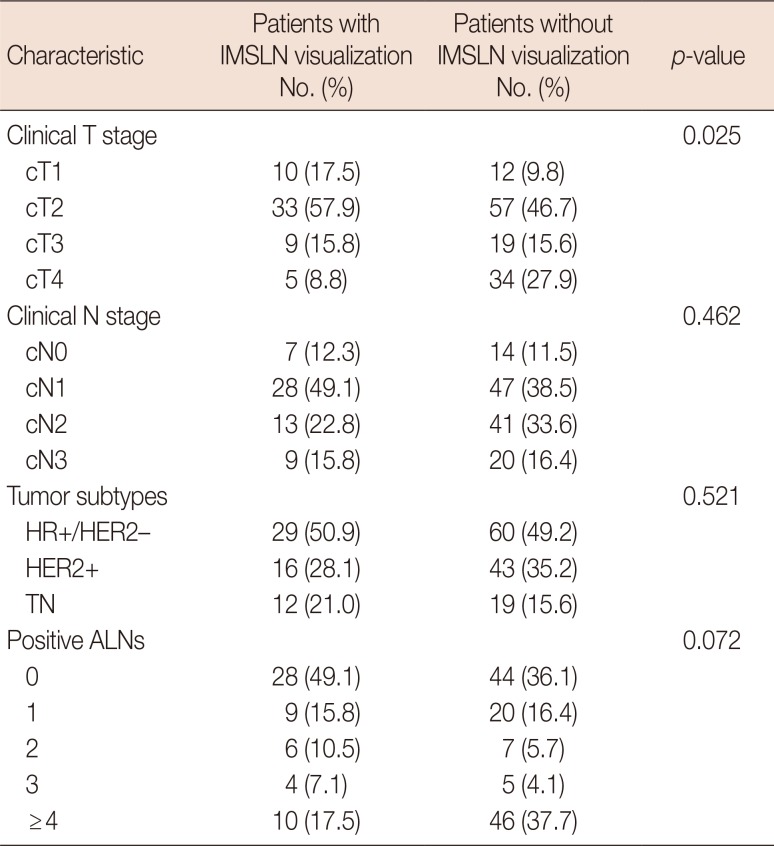
All patients were injected with radiolabeled colloid using modified injection technology. The visualization rate of IMSLN after NAC was 31.8% (57/179), while 31.3% (56/179) of patients underwent IM-SLNB and was 12.3% (7/57) and 87.7% (50/57) among cN0 and cN+ disease, respectively. The success rate of IM-SLNB was 98.2% (56/57). The median number of IMSLN was 1 (total, 87; range, 1–4), one and two IMSLNs were identified in 32 and 15 cases, and three or more IMSLNs were retrieved in nine cases (16.1%). The clinical characteristics and tumor subtype in patients with and without IMSLN visualization are listed in Table 1. The clinical tumor stage was negatively correlated with visualization of IMSLN (p=0.025). Moreover, 45.5%, 36.7%, 32.1%, and 12.8% of patients with cT1, cT2, cT3, and cT4 disease, respectively. Tumor subtype, clinical nodal stage, and the number of positive ALNs had no effect on IMSLN visualization rate (all p>0.05).
IMSLN-positive rate was 7.1% (4/56). All of them coexisted with positive ALNs, and the number of positive ALNs in patients with IMSLN metastasis were 3, 6, 8, and 9. The pathology nodal stage had been changed from pN1/pN2 to pN3b. The pathology stage had been changed from IIA/IIIA to IIIC. The tumor subtype in patients with IMLN metastasis was HR+/HER2−. The positive IMSLN was located in the second intercostal space in two patients and the third intercostal space in two patients. Two patients had complications. One patient had internal mammary artery injury, which was resolved intraoperatively without postoperative bleeding. One patient had pleural injury that was repaired intraoperatively without pneumothorax. The median time-consuming of IM-SLNB was 10 minutes (range, 4–20 minutes).
IMLN and ALN belong to the “first station” lymph node of breast cancer lymphatic drainage, and internal mammary lymphatic vessel is one of the most important metastatic pathways of breast cancer [7]. Suami et al. [8] found that the internal mammary lymphatic vessels were identified alongside the internal mammary blood vessels, deep to the parietal pleura, with IMLNs present in the intercostal spaces. Collecting lymphatics passed through the intercostal muscle beside each perforating blood vessel to join the internal mammary lymphatic system. Moreover, no apparent connection was observed between the collecting lymphatics accompanying the branches of the internal mammary blood vessels and the superficial collecting lymphatics. Li et al. [9] found that the lymphatic density around the breast tumor were reduced significantly after NAC than before, while the density of lymphatic vessels in the breast tumor was not significantly changed. The change of the density in the lymphatic vessels around the tumor was related to the therapeutic effect of NAC. Some investigators reported that chemotherapy could alter the lymphatic drainage patterns by shrinkage to and fibrosis of lymph vessels as well as by obstructing lymphatic channels with cellular material or tumor emboli [1011]. Tsuyuki et al. [12] compared the sentinel lymphatic pathways to the axillary sentinel lymph node (ASLN) and ASLN location before and after NAC in 36 patients (38 breasts) using the indocyanine green-fluorescence method. The result showed that although the locations of ASLNs were not affected by NAC, 42.1% (16/38 affected breasts) of the sentinel lymphatic pathways were changed by NAC. Furthermore, the false-negative rate of A-SLNB was 25% after NAC. However, researches on internal mammary lymphatic pathways after NAC were limited, whether lymphatic vessels that drain into the IMLN were affected by NAC has not been confirmed.
The nodal pCR was identified as no existence of metastatic carcinoma in ALNs and has been shown to be associated with improved survival outcomes. However, the definition of nodal pCR assessed the pathological state of ALN, but overlooked the pathological state of IMLN. As IMLN metastasis has similar prognostic importance as that of ALN, the lymphatic metastasis and staging should involve not only ALN but also IMLN. In patients without receiving NAC, the incidence of IMLN metastasis was 18%–33%, with 2%–11% of patients whose lymph node metastases situated only in IMLN without ALN [13]. It seems to be very meaningful in case of ALN−/IMLN+; however, we have not encountered this situation in patients without NAC. In addition, few researches were conducted with the metastatic rate of IMLN after NAC. A retrospective study on 74 patients who received IM-SLNB after NAC reported that the metastatic rate of IMLN was 7.3% (3/41) [14]. In our study, the metastatic rate of IMLN after NAC was 7.1% (4/56), similar to the study by Cao et al. [14], which were all accompanied by ALN metastases. The number of positive ALNs that patients with IMSLN metastasis was 3, 6, 8, and 9. The pathological nodal stage had been changed from pN1/pN2 to pN3b. The pathological stage had been changed from IIA/IIIA to IIIC. With the increase of sample size, some patients with IMLN metastasis had no ALN metastasis after NAC. Therefore, we should consider the pathological state of IMLN when evaluating the nodal pCR. Therefore, IM-SLNB after NAC should be performed to clarify the whole nodal staging as 7.1% of patients had IMSLN metastases after NAC.
With the continuous maturation of radiotherapy technology, IMLN irradiation that can improve the survival of breast cancer patients has gained more and more attention. The meta-analysis of three clinical trials, i.e., European Organization for Research on Treatment of Cancer (EORTC, n=4,004), NCIC Clinical Trials Group MA.20 trial (n=1,832), and French trials (n=1,334) confirmed that based on the whole breast and the chest wall irradiation, additional IMLN irradiation and supraclavicular region radiotherapy could significantly improve the 10-year overall survival (hazard ratio, 0.88; 95% confidence interval [CI], 0.78–0.99) and 10-year disease-free survival (hazard ratio, 0.86; 95% CI, 0.78–0.95) [15]. As no stage III clinical trials could support irradiation in patients after NAC, the 2017 National Comprehensive Cancer Network Breast Cancer Guidelines recommended that adjuvant radiation therapy post-BCS or postmastectomy was based on prechemotherapy tumor staging because radiation therapy to chest wall plus infraclavicular region, supraclavicular area, IMLN irradiation, and any part of the axillary bed at risk in patients with ≥4 positive ALNs (category 1)\strongly suggest IMLN irradiation in patients with 1 to 3 positive ALNs (category 2A) [16].
At present, the indication of IMLN irradiation mainly depends on the high-risk factors of IMLN metastases (only patients with high-risk metastasis factors were selected without IMLN pathology assessment) [17]. Most patients who received NAC were in the advanced stage with high-risk factors of IMLN metastases. However, patients with high-risk factors of IMLN metastases do not necessarily have IMLN metastases, and low-risk patients cannot be excluded of the possibility of IMLN metastases. Some patients with negative IMLN would receive overtreatment, and those with positive IMLN but negative ALN would receive undertreatment. Therefore, the pathological status of IMLN is superior to the high-risk factors for the guidance of IMLN irradiation, and IM-SLNB could help identify the metastasis of IMLN. In patients without NAC, IMLN irradiation could be avoided in patients with negative IMSLN [14], whereas whether it is appropriate in patients with NAC should be further explored.
The IMLN dissection provided the main data in assessing the pathological status of IMLN. However, the extended radical mastectomy had been abandoned because it cannot improve the overall patient survival [13]. IMLN has been well known to usually have a deep anatomical position and small diameter (<0.5 cm), and the sensitivity of imaging examination cannot meet the clinical requirements to detect IMLN metastases. With the development of SLNB technique, IM-SLNB is expected to be a minimally invasive diagnostic technique in assessing the status of IMLN metastasis [18]. However, IMSLN can be identified only in a small proportion of patients with the tradition injection technique, which has been the biggest restriction of IM-SLNB. Based on the hypothesis that IMSLN receives lymphatic drainage not only from the primary tumor area but also the entire breast parenchyma, Qiu et al. [619] injected radiotracer using modified injection technique (periareolar intraparenchymal, high volume, and ultrasound guidance), which could significantly improve the preoperative visualization rate of the IMSLN. With the combination of the intraoperative gamma probe, the detection rate of IMSLN could reach 77.2% (p<0.05).
Cao et al. [14] evaluated the data of 74 breast cancer patients who received NAC. Results showed that with the guidance of modified injection technique, the visualization rate of IMSLN after NAC was 56.8% (42/74). These numbers are promising, but are too small. From November 2011 to 2017, 179 breast cancer patients who underwent NAC were enrolled in our study, with the guidance of modified injection technique, and the visualization rate of IMSLN was 31.8% (57/179). The clinical tumor stage could affect the visualization rate of IMSLN after NAC. Although a difference in IMSLN visualization rate was found between different clinical nodal stages, the difference was not significant.
In our study, among the 57 patients with radioactive IMSLN, 56 received IM-SLNB successfully, and its success rate was 98.2%. Only two patients (3.6%) had complications, which were in the acceptable range. Therefore, IM-SLNB is a safe and feasible minimally invasive technique and should be recognized and taken into practice.
IM-SLNB after NAC should be considered and taken into practice, especially in patients with cN+ disease, in order to complete lymph nodal staging. IM-SLNB could further improve the definition of nodal pCR and guide internal mammary node irradiation.
Notes
References
1. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997; 15:2483–2493. PMID: 9215816.

2. Li XB, Krishnamurti U, Bhattarai S, Klimov S, Reid MD, O'Regan R, et al. Biomarkers predicting pathologic complete response to neoadjuvant chemotherapy in breast cancer. Am J Clin Pathol. 2016; 145:871–878. PMID: 27298399.

3. Caudle AS, Yi M, Hoffman KE, Mittendorf EA, Babiera GV, Hwang RF, et al. Impact of identification of internal mammary sentinel lymph node metastasis in breast cancer patients. Ann Surg Oncol. 2014; 21:60–65. PMID: 24046126.

4. Kumar A, Puri R, Gadgil PV, Jatoi I. Sentinel lymph node biopsy in primary breast cancer: window to management of the axilla. World J Surg. 2012; 36:1453–1459. PMID: 22555287.

5. Cong BB, Qiu PF, Wang YS. Internal mammary sentinel lymph node biopsy: minimally invasive staging and tailored internal mammary radiotherapy. Ann Surg Oncol. 2014; 21:2119–2121. PMID: 24664625.

6. Qiu PF, Cong BB, Zhao RR, Yang GR, Liu YB, Chen P, et al. Internal mammary sentinel lymph node biopsy with modified injection technique: high visualization rate and accurate staging. Medicine (Baltimore). 2015; 94:e1790. PMID: 26469922.
7. Irigo M, Coscarelli L, Rancati A. Anatomical basis of pedicles in breast reduction. Gland Surg. 2017; 6:154–162. PMID: 28497019.

8. Suami H, Pan WR, Mann GB, Taylor GI. The lymphatic anatomy of the breast and its implications for sentinel lymph node biopsy: a human cadaver study. Ann Surg Oncol. 2008; 15:863–871. PMID: 18043970.

9. Li D, Chen JH, Yao Q, Yang H, Wang L. Determination of lymphatic vessel density in breast cancer patients receiving neoadjuvant chemotherapy. China Oncol. 2007; 17:754–757.
10. Kuerer HM, Hunt KK. The rationale for integration of lymphatic mapping and sentinel node biopsy in the management of breast cancer after neoadjuvant chemotherapy. Semin Breast Dis. 2002; 5:80–87.
11. Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol. 1996; 9:893–900. PMID: 8878021.
12. Tsuyuki S, Yamaguchi A, Kawata Y, Kawaguchi K. Assessing the effects of neoadjuvant chemotherapy on lymphatic pathways to sentinel lymph nodes in cases of breast cancer: usefulness of the indocyanine green-fluorescence method. Breast. 2015; 24:298–301. PMID: 25802085.

13. van der Ent FW, Kengen RA, van der Pol HA, Povel JA, Stroeken HJ, Hoofwijk AG. Halsted revisited: internal mammary sentinel lymph node biopsy in breast cancer. Ann Surg. 2001; 234:79–84. PMID: 11420486.

14. Cao XS, Li HJ, Cong BB, Sun X, Qiu PF, Liu YB, et al. Axillary and internal mammary sentinel lymph node biopsy in breast cancer after neoadjuvant chemotherapy. Oncotarget. 2016; 7:74074–74081. PMID: 27738336.

15. Budach W, Kammers K, Boelke E, Matuschek C. Adjuvant radiotherapy of regional lymph nodes in breast cancer: a meta-analysis of randomized trials. Radiat Oncol. 2013; 8:267. PMID: 24225206.

16. National Comprehensive Cancer Network. Breast cancer, version 3.2017 featured updates to the NCCN guidelines. Accessed November 10th, 2017. Available from: http://www.nccn.org.
17. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008; 107:379–387. PMID: 17457670.

18. Bi Z, Qiu PF. Advances in diagnosis and treatment of internal mammary lymph node of breast cancer. Chin J Clin Oncol. 2017; 44:1104–1107.
19. Qiu PF, Liu YB, Wang YS. Internal mammary sentinel lymph node biopsy: abandon or persist? Onco Targets Ther. 2016; 9:3879–3882. PMID: 27390528.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download