Abstract
Purpose
T-cell immunoglobulin and mucin domain-containing molecule 3 (TIM-3) is an emerging immune response molecule related to T-cell anergy. There has been tremendous interest in breast cancer targeting immune checkpoint molecules, especially in the triple-negative breast cancer (TNBC). This study was designed to investigate TIM-3 expression on tumor infiltrating lymphocytes (TILs), its relationships with clinicopathological para-meters and expression of programmed death receptor 1 (PD-1)/programmed death receptor ligand 1 (PD-L1), and its prognostic role.
Methods
Immunohistochemistry on tissue microarray blocks produced from 109 samples of invasive ductal carcinoma type TNBC was performed with antibodies toward TIM-3, PD-1, PD-L1 and breast cancer-related molecular markers. Associations between their expression and clinicopathological parameters as well as survival analyses were performed.
Results
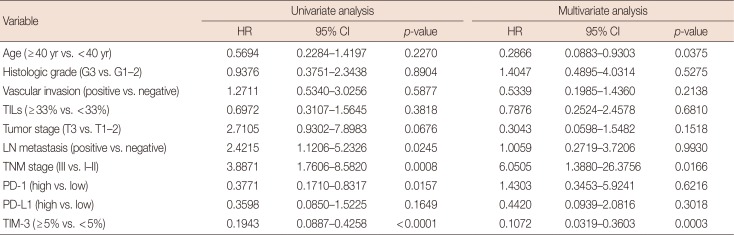
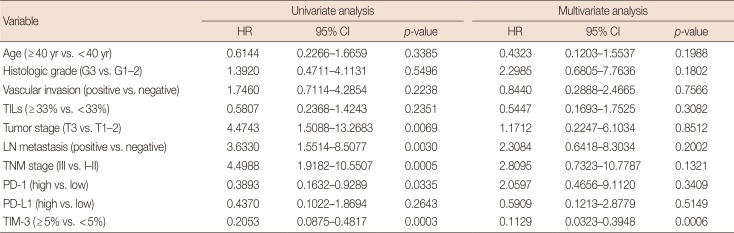
TIM-3 was expressed in TILs from all 109 TNBCs, consisting of 17 cases (<5%), 31 cases (6%–25%), 48 cases (26%–50%), and 13 cases (>51%). High TIM-3 was significantly correlated with younger patients (p=0.0101), high TILs (p=0.0029), high tumor stage (p=0.0018), high PD-1 (p=0.0001) and high PD-L1 (p=0.0019), and tended to be associated with higher histologic grade, absence of extensive in situ components and microcalcification. High TIM-3 expression was significantly associated with a combinational immunophenotype group of high PD-L1 and high PD-1 (p<0.0001). High TIM-3 demonstrated a significantly better disease-free survival (DFS) (p<0.0001) and longer overall survival (OS) (p=0.0001), together with high TILs and high PD-1. In univariate survival analysis, high TIM-3 showed reduced relapse risk (p<0.0001) and longer OS (p=0.0003), together with high PD-1 expression. In multivariate analysis, high TIM-3 was statistically significant in predicting prognosis, showing better DFS (hazard ratio [HR], 0.0994; 95% confidence interval [CI], 0.0296–0.3337; p=0.0002) and longer OS (HR, 0.1109; 95% CI, 0.0314–0.3912; p=0.0006).
Triple-negative breast cancer (TNBC) accounts for 15% to 20% of all breast cancers. TNBC lacks expression of estrogen receptor (ER) and progesterone receptor (PR) and has normal human epidermal growth factor receptor 2 (HER2) gene copy number and expression [1]. TNBC affects younger women more frequently and has a worse prognosis than breast cancer in general due to a combination of more aggressive clinical behavior and a lack of molecular targets for therapy [2]. Recently, attention toward the complex role of the immune system in cancer growth, metastasis and elimination has increased. More detailed analyses of the intricate roles of the constituents of the immune system could reveal potential prognostic or predictive markers for cancer progression and treatment targets for a group of refractory cancers including TNBC [2].
Although breast cancer is one of least immunogenic tumors in general, TNBC and HER2 positive breast cancers are more immunogenic than luminal type breast cancer. Tumor infiltrating lymphocytes (TILs) have been shown to be a strong prognostic indicator in TNBC in particular but also in general breast cancer. Several studies demonstrated that in TNBCs, increased TILs within the tumor microenvironment were associated with a reduction in relative risk of distant recurrence or death [3]. Regarding the components of TILs, T-lymphocytes are the most predominant type of lymphocytes in the tumor microenvironment, constituting up to 75% and cytotoxic CD8+ T lymphocytes play a key role in the adaptive immunological defense against cancer cells, following reported associations with better prognosis [345]. During the past decade, new insights into the control of T-cell activation and proliferation have led to the identification of checkpoint proteins that either up- or downregulate T-cell reactivity [67]. Approaches to break effector T-cell anergy and to block suppressive cell types and ligands to eradicate cancer led to monoclonal antibody immunotherapies that inhibit cytotoxic T lymphocyte antigen 4 or programmed death receptor 1 (PD-1) on T-cells or programmed death receptor ligand 1 (PD-L1) present at cancer cell membranes; all of which have become promising molecular targets for anticancer immunotherapy, especially in highly immunogenic cancers such as non-small cell lung cancer, malignant melanoma, and renal cell carcinoma [8].
T-cell immunoglobulin and mucin domain 3 (TIM-3), a member of the TIM family, is an immune checkpoint molecule and has been shown to be expressed on CD4+ T-helper 1 (Th1) cells, CD8+ T-cells, dendritic cells, other lymphocyte subsets, subpopulations of macrophages and monocytes [9], and recently cancer cells [1011]. Binding with galectin-9, TIM-3 induces Th1 cell death, suggesting its function in the negative regulation of Th1 response [12]. TIM-3 has been demonstrated as an important regulator of CD8+ T-cell exhaustion in cancer [13]. The exhaustion of T-cells induces T-cell dysfunction in the immune response and thus prevents optimal control of tumors. On the contrary, TIM-3 downregulation in tumor cells has been suggested as a prognosticator for cancer progression in colorectal and prostate cancer [1011]. Now, TIM-3 is one of several immune checkpoint inhibitory molecules and one of critical mediator in cancer progression that are attracting attention as a target, but it remains relatively poorly studied in oncoimmunology.
According to emerging evidence that the constituents of the immune system will have potential as prognostic or predictive markers of cancer progression as well as treatment targets for a wide range of tumors, the identification of immune-related factors in heterogeneous TNBC is a clinically unmet need. In this study, we performed TIM-3 expression profiling of TILs in TNBC using immunohistochemistry (IHC) and analyzed correlations with clinicopathological factors, molecular biomarkers and expressions of PD-L1 in cancer cells and PD-1 in TILs following survival analyses with the aim to identify whether TIM-3 expression levels in TILs act as a prognosticator.
Medical records which include pathologic diagnosis of breast carcinoma and surgical resection were searched between 2007 and 2011 at Dong-A University Hospital, Busan, Korea. After histologic and molecular review of a total of 837 cases, we selected 109 cases of TNBC which represented ER(−), PR(−) and HER2(−) showing normal expression or no amplification with a histologic type of invasive ductal carcinoma that were available for our investigation. All patients did not receive any neoadjuvant chemotherapy before surgery. The degree of TILs was initially evaluated based on the percentage of tumor stromal area occupied by TILs, according to the suggestion of the International TILs Working Group [14]. TILs scores were re-classified into three groups (≤5%, 6%–33%, ≥34%). Patient details and clinical information, including age at initial diagnosis, histologic grade, tumor stage, lymph node metastasis, TNM stage, date of surgery, date of last follow-up, and date of recurrence or death were evaluated retrospectively from the electronic medical records. The median follow-up time was 76 months (range, 6–131 months) and the median age of patients at diagnosis was 50 years (range, 30–74 years). Furthermore, disease-free survival (DFS) was defined as the duration from the date of initial diagnosis to the first detection of breast cancer-specific relapse or death. Overall survival (OS) was defined as the time interval from the date of initial diagnosis to the date of breast cancer-related death. The clinicopathological information of TNBC patients is given in Table 1. This study was approved by the Institutional Review Board at the Dong-A University Hospital, Busan, Korea (DAUHIRB-18-051) and was exempt from patient informed consent due to use of archived formalin-fixed and paraffin-embedded (FFPE) tumor tissue.
We prepared a tissue microarray (TMA), possessing 109 TNBC cancer tissue punches from FFPE tumor samples according to a previously described format [15]. Numerous cancer cell areas with dominant TILs on hematoxylin and eosin stained slides were identified and two 3-mm tissue cores from individual tumors were obtained. TMAs were constructed with a tissue arrayer (Unitma Co., Ltd., Seoul, Korea). Ten TMA blocks were constructed.
IHC for TIM-3, PD-L1 and PD-1 was performed with 4-µm thick TMA tissue sections by using a BenchMark XT automated immunostainer (Ventana Medical System Inc., Tuscon, USA). After deparaffinization, rehydration and antigen retrieval, diluted primary TIM-3 rabbit monoclonal antibody (1:100; D5D5R™; Cell Signaling Technology, Beverly, USA), PD-L1 rabbit monoclonal antibody (1:100; E1L3N; Cell Signaling Technology) and PD-1 mouse monoclonal antibody (1:50; NAT105; Abcam, Cambridge, UK) were incubated. The primary antibodies were detected with Ultraview Universal DAB Detection Kit (Ventana Medical System Inc.), according to the manufacturer's instructions, followed by hematoxylin counterstaining. For the validation of these antibodies, we used tonsillar tissue for TIM-3 antibody and PD-1 antibody, and placental tissue for PD-L1 antibody as positive controls. Two independent pathologists (J.S.J. and M.H.J.) observed the slides in a blinded manner. The distribution of TIM-3 expression in TNBC was identified as the percentage of distinctly immune-stained TILs among total TILs, as represented in Figure 1 and divided into score 1 (≤5%), 2 (6%–25%), 3 (26%–50%) and 4 (≥51%), referring to a previous study [16]. For the evaluation of PD-L1 expression of TNBC cancer cells, we assessed the staining intensity with a 4-tiered scoring consisting of negative (0), weak (score 1), moderate (score 2) and strong (score 3) as well as the distribution of stained cancer cells by percentage, finally multiplying intensity score by distribution percentage to obtain the expression score (range, 0–300). By using a modified Muenst's scoring method [17], PD-L1 expression was categorized into two groups according to the final scores: low expression (<100) and high expression (≥100). According to the distribution of PD-1 expression in TILs, scoring was divided into 0 (≤5%), 1 (6%–33%), 2 (34%–66%) and 3 (>66%) and re-categorized into a low expression group (score 0 and 1) and high expression group (score 2 and 3).
The IHC study for the expression of ER (1:50), PR (1:50), HER2 (1:200), Ki-67 (1:800), cytokeratin 5/6 (1:50), epidermal growth factor receptor (EGFR) (1:50) and p53 (1:1,200) was available in all tissues. The interpretation of staining intensity and distribution for ER, PR, and HER2 expression was analyzed as described previously [18].
The MedCalc software program (version 18.2.1; MedCalc, Ostend, Belgium) was used for statistical analyses. The distributions of TIM-3 expression levels in TILs with clinicopathological characteristics and biomarkers were compared using the chi-square test. DFS and OS based on TIM-3 expression were assessed by the Kaplan-Meier method with the log-rank test. Univariate survival analysis was performed in individual covariate and multivariate survival analyses using the Cox proportional hazards regression model to assess whether the expression level of TIM-3 in TILs was an independent predictor of disease relapse or survival. In all of the tests, p≤0.05 was defined as statistically significant.
A total of 109 primary TNBC patients were included in this study. The median patient age was 50 years (range, 30–74 years). The mean tumor size was 2.5±1.7 cm. Four cases (3.7%) were histologic grade 1, 21 cases (19.3%) were grade 2, and 84 cases (77.1%) were grade 3. Details of the clinicopathological features of this cohort are summarized in Table 1. TIM-3 was expressed in mononuclear leukocytes, which were mainly TILs, and some histiocytoid cells (Figure 1) and cancer cells (data not shown). In this study, we analyzed TIM-3 expression in nonneoplastic immune cells. Overall, 17 cases (15.6%) were score 1, 31 cases (28.4%) were score 2, 48 cases (44.0%) were score 3 and 13 cases (11.9%) were score 4. Ninety cases (82.6%) were low PD-L1 and 19 cases (17.4%) were high PD-L1. Sixty cases (55.0%) were low PD-1 and 49 cases (45.0%) were high PD-1.
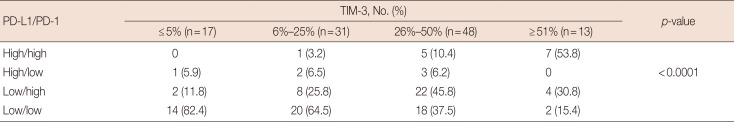
High TIM-3 expression was significantly correlated with younger patients (p=0.0101), high levels of TILs (p=0.0029) and tumor stage (p=0.0018). Higher histologic grade, absence of extensive in situ components and absence of microcalcification tended to be related with high TIM-3 expression, although not statistically significant (p=0.2101, p=0.0706, p=0.1174, respectively). There were no correlations with CK5/6, EGFR, Ki-67 and p53 expression, lymph node metastasis stage or TNM stage. High TIM-3 expression was closely correlated with increased PD-L1 expression in cancer cells (p=0.0019) and high PD-1 expression in TILs (p=0.0001). The results are summarized in Table 1. Furthermore, a correlation between TIM-3 expression levels and combinational immunophenotypes of PD-L1 and PD-1 expression was observed. High TIM-3 expression was significantly associated with the high PD-L1/high PD-1 group (p<0.0001) (Table 2).
Follow-up information was available in all 109 patients with a median follow-up period of 76 months (range, 6–131 months). Disease relapse was observed in 26 patients (23.9%) and 22 patients (20.2%) died. To examine whether TIM-3 expression level in TILs has a prognostic value for DFS and OS in TNBC, Kaplan-Meier curves of high and low TIM-3 expression groups were constructed and are presented in Figure 2A. In this plot, patients with high TIM-3 expression had a significantly better prognosis (DFS, p<0.0001; OS, p=0.0001). Patients with high TILs were also associated with better DFS (p=0.0587) and OS (p=0.0167) (Figure 2B). High PD-L1 expression in cancer cells tended to be related with better DFS (p=0.1461) and OS (p=0.2502), although not statistically significant (Figure 2C), and high PD-1 expression in TILs showed better DFS (p=0.0118) and OS (p=0.0272) (Figure 2D).
Furthermore, we generated univariate and multivariate analyses with the Cox proportional regression hazards model in our database to investigate whether TIM-3 expression was an independent prognostic factor for predicting DFS and OS in TNBC. In univariate survival analysis, TNBCs with high TIM-3 expression showed significantly reduced relapse risk (p<0.0001) and better OS (p=0.0003) (Tables 3 and 4). Patients with high PD-1 expression in TILs were also associated with better DFS (p=0.0157) and OS (p=0.0335) (Tables 3 and 4). High tumor stage, presence of nodal metastasis and advanced tumor stage were significantly related with risk of relapse (p=0.0676, p=0.0245, and p=0.0008, respectively) and worse OS (p=0.0069, p=0.0030, and p=0.0005, respectively) (Tables 3 and 4). Next, multivariate analysis involving all variables that were tested in univariate analysis was generated. High TIM-3 expression in TILs of TNBC patients were statistically significant in predicting prognosis, showing better DFS (hazard ratio [HR], 0.1072; 95% confidence interval [CI], 0.0319–0.3603; p=0.0003) and longer OS (HR, 0.1129; 95% CI, 0.0323–0.3948; p=0.0006) (Tables 3 and 4). Young patients and advanced stage were also significantly associated with risk of relapse (Table 3).
In this study, we found that high TIM-3 expression in TILs was significantly associated with better DFS and OS in the triple-negative subtype of invasive ductal carcinoma (IDC). Further, through multivariate analysis, high TIM-3 expression in TILs of TNBC was an independent prognostic factor associated with longer recurrence-free survival and longer OS, despite the fact that it was associated with poor clinicopathological factors. These results suggest that high TIM-3 expression in TILs might translate into a good prognostic biomarker for relapse-free survival and OS in TNBC.
There are very few papers on TIM-3 expression in breast cancer and not many in other cancers. We searched the literature for the relationship between TIM-3 expression and clinicopathological factors in breast cancer and found one report. Zhang et al. [19] reported that TIM-3+/CD8+ T-cells in 150 IDC patients were correlated with lymph node metastasis, histologic grade, and molecular classification similar to our study, but there was no survival analysis. The difference from our study was that Zhang et al. [19] observed the expression of TIM-3 on CD8+ T-cells, but we observed it on overall TILs, while their study was on IDC, but our study was on triple-negative subtype among IDC.
In addition to breast cancer, upregulated TIM-3 on either CD4+ and CD8+ TILs or overall TILs have been reported in lung cancer [20], hepatocellular carcinoma (HCC) [21], head and neck cancer [22], and so forth. Poor clinicopathological factors were correlated with high TIM-3 expression, but the observed cells were diverse, representing CD4+ TILs but not CD8+ TILs [20], overall TILs [21] or CD8+ TILs [22]. Patient survival data for high TIM-3 expression were limited and inconsistent, being reported as a negative prognostic factor of OS in HCC [21] or no relation in head and neck cancer [22]. Furthermore, even upregulated TIM-3 on overall TILs in HCC was related with poor clinicopathological factors and poor survival; it was demonstrated that TIM-3+ CD4+ T-cells had reduced proliferation and activation potential in HCC [21].These results may be inferred from the range of covered TILs, detection methods, qualified analyses in case of immunohistochemistry, cancer type and subtype and so on, and also from the reality that TIM-3 remains relatively poorly studied in oncology.
TIM-3 was originally identified by expression on Th1-specific cells and subsequently on a subset of activated CD4+ T-cells, differentiated Th1 cells, CD8+ T-cells and Th17 cells as well as on cells of the innate immune system including mast cells, subpopulations of macrophages and dendritic cells, natural killer cells, monocytes [9], and also cancer cells [1011]. The clinical impact of TIM-3 being one of many similar inhibitory molecules for negative regulation of cytotoxic T-cells reveals new targets in immunotherapy field of cancer treatment, but there are also reports of conflicting studies by cell types; thus, it is suggested that TIM-3 may play different biological roles in different leukocyte subsets [22]. Here, we suppose that TIM-3 expression in overall TILs will be important in the development of an approach for cancer prognosis, in addition to unraveling the roles of TIM-3 in individual cellular subsets.
Meanwhile, there have been recent studies related to TIM-3 expression in cancer cells and clinical outcome. These studies also showed contradictory results, such as a poor prognosis of low TIM-3 in prostate cancer [12] and mediation of colorectal cancer progression by upregulation of TIM-3 [13].
Breast cancer is not generally immunogenic, but its association with the immune system is well-documented by the prognostic impact of TILs and the expression of immune gene signatures in some breast cancers [23]. The role of TILs as a prognostic marker has been evaluated in several large studies and consistently showed that in TNBC patients, TILs were associated with an improved clinical outcome [23]. Our results also show that high TILs are correlated with better DFS and OS. In subsequent studies, cytotoxic CD8+ T lymphocytes were shown play a key role in the adaptive immunological defense against cancer cells and were reported to be associated with a better prognosis [345]. PD-1 expressed on activated T-cells, B-cells, natural killer cells, and myeloid cells conveys an inhibitory signal to T-cells and thus impedes immune responses regulated by the PD-L1–PD-1 axis [78]. In this regard, evaluation of PD-1+ TILs could be important, but information on PD-1+ TILs in breast cancer is limited; a study reported its association with higher histologic grade, negative ER and high TILs, but failed to demonstrate an independent prognostic role [23]. In our study, high PD-1 was associated with high TIM-3 expression and associated with longer DFS and OS, but was not an independent prognosticator. In breast cancer, the frequency of PD-L1 expression by cancer cells has been reported to vary widely, ranging from 1.7% to over 50% [17242526], with more frequent expression reported in TNBC [172425], as with our result, as well as in HER2 positive cancers [26]. In our study, the apparent PD-L1 expression was 17.4% (19 cases) of TNBC. Moreover, conflicting results have been pub-lished regarding the clinicopathological relationship and prognostic value of PD-L1 in breast cancer [172426]. In our study, PD-L1 was not associated with disease-free or overall survival, although we found a positive correlation between TIM-3 expression in TILs with PD-L1 expression in cancer cells and PD-1 expression in TILs, as well as the degree of TILs.
Furthermore, our study demonstrated that higher TIM-3 expression in TILs was significantly associated with a combinational immunophenotype of high PD-L1 and high PD-1 compared with other combinations. Our results showed that TIM-3 and the combinational immunophenotype of PD-L1 and PD-1 did not show any significant associations with relapse-free and overall survival (data not shown) compared with a previous study representing worse survival in low PD-1 TILs/high PD-L1 group of HER2 positive breast cancer [26]. Also, individual immune checkpoint inhibitors appear to be a potentially promising target for cancer immunotherapy; current studies reported that a simultaneous blockade of two or more inhibitors leads to more inhibition of tumor growth than single inhibitor treatment alone [27]. In fact, we understand that our study as well as other published studies have some limitations, such as the fact that the use of TMAs may not accurately represent expression due to intra-tumoral heterogeneity and aspects regarding the reliability of IHC staining for those inhibitors due to the lack of a standardized staining method and analysis protocol as well as the variety of antibodies. However, according to recent trends in the field of immuno-oncology research, we believe that pattern studies of these immune checkpoint inhibitors, alone or in combinations, should be continued indispensably to search for potential candidates for prognostic factors as well as therapeutic targets in cancer.
Despite its association with poor clinical and pathologic features and it being a currently emerging target molecule for anticancer immunotherapy via blockade, we demonstrate that TIM-3 expression in TILs is an independent positive prognostic biomarker in TNBC. Research for further characterization of TIM-3+ TILs and large-scale cohort studies for validation should certainly be pursued.
ACKNOWLEDGMENTS
We wish to thank to prof. Jin Ho Chun, MD, PhD in the Department of Preventive Medicine, Inje University College of Medicine, Busan, Korea for helping us to use the medical statistics method, to analyse and to interpret the clinical significance.
References
1. Liedtke C, Gonzalez-Angulo AM, Pusztai L. Definition of triple-negative breast cancer and relationship to basal-like molecular subtype. In : DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 9th ed. Philadelphia: Lippincott Williams & Wilkins;2011. p. 1–6.
2. Stovgaard ES, Nielsen D, Hogdall E, Balslev E. Triple negative breast cancer: prognostic role of immune-related factors: a systematic review. Acta Oncol. 2018; 57:74–82. PMID: 29168430.
3. Ibrahim EM, Al-Foheidi ME, Al-Mansour MM, Kazkaz GA. The prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancer: a meta-analysis. Breast Cancer Res Treat. 2014; 148:467–476. PMID: 25361613.

4. Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011; 29:1949–1955. PMID: 21483002.

5. Matsumoto H, Thike AA, Li H, Yeong J, Koo SL, Dent RA, et al. Increased CD4 and CD8-positive T cell infiltrate signifies good prognosis in a subset of triple-negative breast cancer. Breast Cancer Res Treat. 2016; 156:237–247. PMID: 26960711.

6. Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, auto-immune disease, and persistent infections. Immunol Rev. 2009; 229:67–87. PMID: 19426215.

7. Melero I, Hervas-Stubbs S, Glennie M, Pardoll DM, Chen L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat Rev Cancer. 2007; 7:95–106. PMID: 17251916.

8. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012; 366:2443–2454. PMID: 22658127.

9. Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyff RH. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010; 235:172–189. PMID: 20536563.
10. Wu J, Lin G, Zhu Y, Zhang H, Shi G, Shen Y, et al. Low TIM3 expression indicates poor prognosis of metastatic prostate cancer and acts as an independent predictor of castration resistant status. Sci Rep. 2017; 7:8869. PMID: 28827755.

11. Yu M, Lu B, Liu Y, Me Y, Wang L, Zhang P. Tim-3 is upregulated in human colorectal carcinoma and associated with tumor progression. Mol Med Rep. 2017; 15:689–695. PMID: 28035413.

12. Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005; 6:1245–1252. PMID: 16286920.

13. Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010; 207:2175–2186. PMID: 20819923.

14. Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015; 26:259–271. PMID: 25214542.

15. Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001; 195:72–79. PMID: 11568893.
16. Soo RA, Kim HR, Asuncion BR, Fazreen Z, Omar MF, Herrera MC, et al. Significance of immune checkpoint proteins in EGFR-mutant non-small cell lung cancer. Lung Cancer. 2017; 105:17–22. PMID: 28236980.
17. Muenst S, Schaerli AR, Gao F, Däster S, Trella E, Droeser RA, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014; 146:15–24. PMID: 24842267.

18. Jang SH, Lee JE, Oh MH, Lee JH, Cho HD, Kim KJ, et al. High EZH2 protein expression is associated with poor overall survival in patients with luminal A breast cancer. J Breast Cancer. 2016; 19:53–60. PMID: 27066096.

19. Zhang H, Xiang R, Wu B, Li J, Luo G. T-cell immunoglobulin mucin-3 expression in invasive ductal breast carcinoma: clinicopathological correlations and association with tumor infiltration by cytotoxic lymphocytes. Mol Clin Oncol. 2017; 7:557–563. PMID: 28855989.

20. Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, et al. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012; 7:e30676. PMID: 22363469.

21. Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology. 2012; 56:1342–1351. PMID: 22505239.

22. Liu JF, Ma SR, Mao L, Bu LL, Yu GT, Li YC, et al. T-cell immunoglobulin mucin 3 blockade drives an antitumor immune response in head and neck cancer. Mol Oncol. 2017; 11:235–247. PMID: 28102051.

23. Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: implication for immunotherapy. BMC Cancer. 2008; 8:57. PMID: 18294387.

24. Bae SB, Cho HD, Oh MH, Lee JH, Jang SH, Hong SA, et al. Expression of programmed death receptor ligand 1 with high tumor-infiltrating lymphocytes is associated with better prognosis in breast cancer. J Breast Cancer. 2016; 19:242–251. PMID: 27721873.

25. Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Hum Pathol. 2016; 47:78–84. PMID: 26541326.

26. Tsang JY, Au WL, Lo KY, Ni YB, Hlaing T, Hu J, et al. PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat. 2017; 162:19–30. PMID: 28058578.
27. Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: detection, characterization and clinical implication. Breast Cancer. 2017; 24:3–15. PMID: 27138387.

Figure 1
Representative T-cell immunoglobulin and mucin domain-3 (TIM-3) expressions in triple-negative breast cancer by immunohistochemistry (×400). (A) Occasionally, stromal tumor infiltrating lymphocytes (TILs) express TIM-3, analyzing into score 1 (≤5%). (B) A few TILs express TIM-3, analyzing into score 2 (6%–25%). (C) Some TILs and histiocytoid cells express TIM-3, analyzing into score 3 (26%–50%). (D) Many TILs and histiocytoid cells express TIM-3, analyzing into score 4 (≥51%).
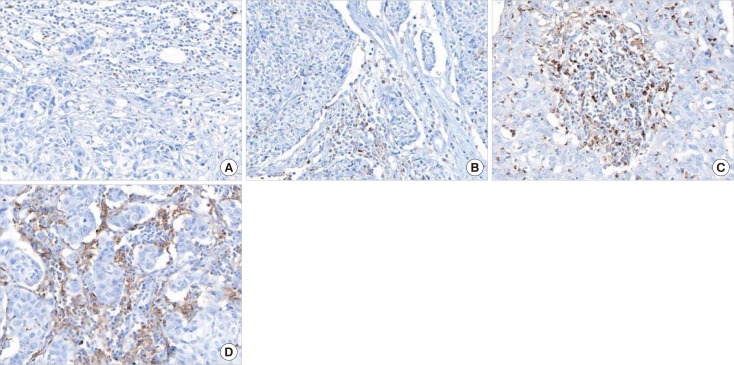
Figure 2
Kaplan-Meier survival curves for disease-free survival (DFS) and overall survival (OS) depending on T-cell immunoglobulin and mucin domain-3 (TIM-3) expression, tumor infiltrating lymphocytes (TILs), programmed death receptor ligand 1 (PD-L1) expression and programmed death receptor 1 (PD-1) expression. (A) High TIM-3 shows significantly better DFS (p<0.0001) and OS (p=0.0001). (B) High TILs show significantly better DFS (p=0.0587) and OS (p=0.0167). (C) High PD-L1 tends to be related with better DFS (p=0.1461) and OS (p=0.2502), statistically insignificant. (D) High PD-1 shows significantly better DFS (p=0.0118) and OS (p=0.0272).
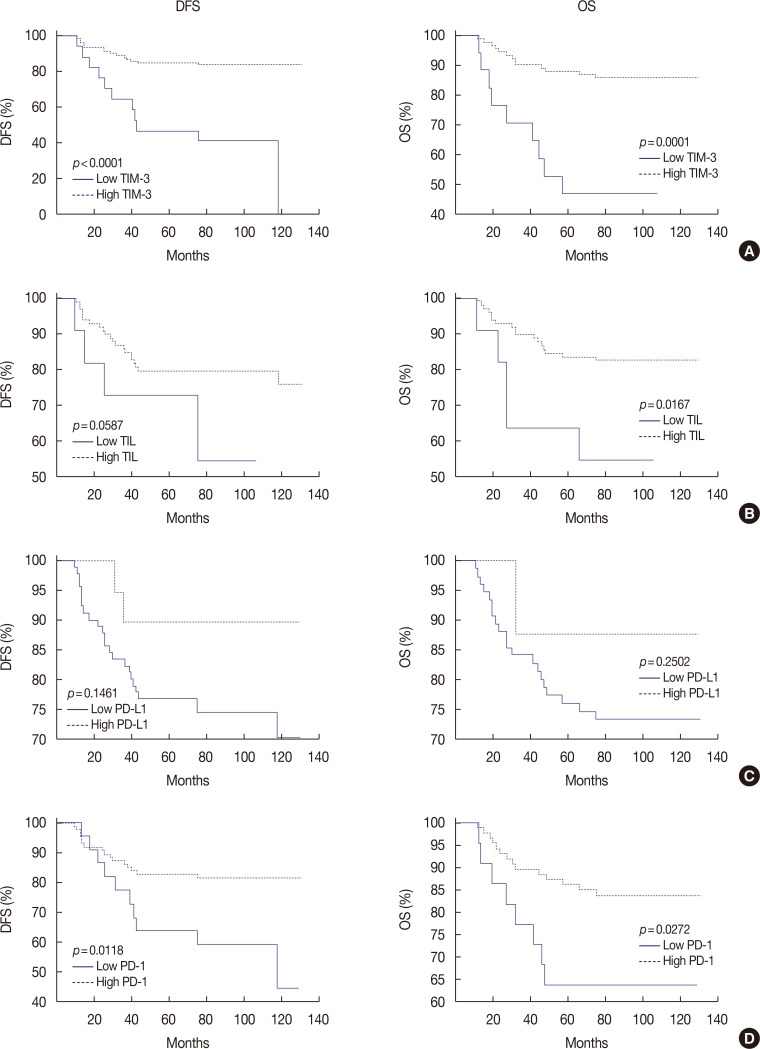
Table 1
Correlation of TIM-3 expression with clinicopathological parameters and PD-1/PD-L1 expressions in TNBC
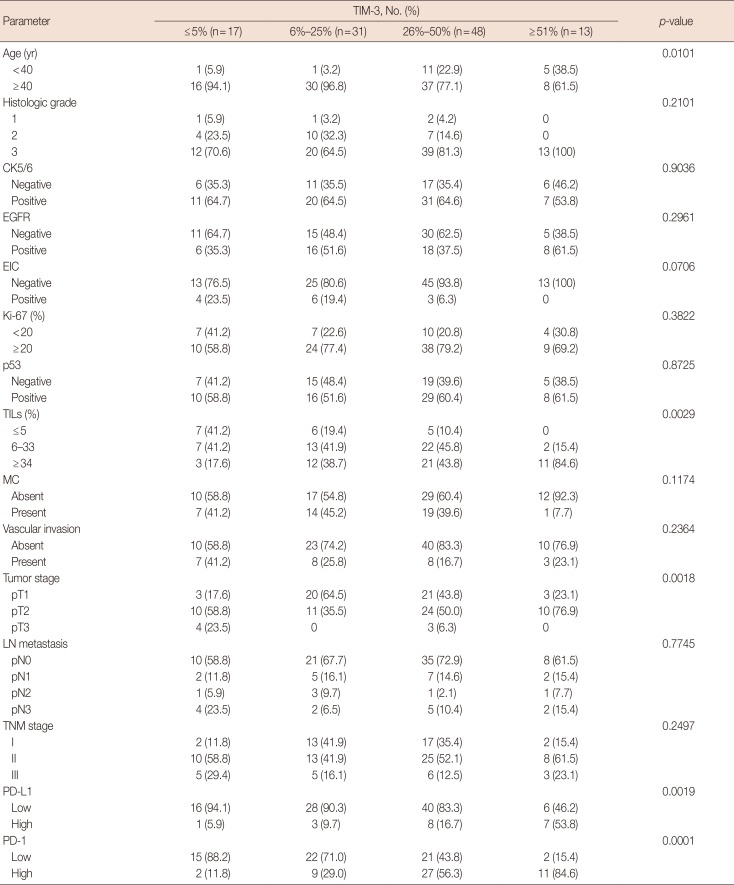
TIM-3=T-cell Immunoglobulin and mucin domain-3; PD-1=programmed death receptor 1; PD-L1=programmed death receptor ligand 1; TNBC=triple-negative breast cancer; CK=cytokeratin; EGFR=epidermal growth factor receptor; EIC=extensive in situ component; TILs=tumor infiltrating lymphocytes; MC=microcalcification; LN=lymph node.
Table 2
Correlation of TIM-3 expression with combinational immunophenotypes of PD-L1/PD-1 expression in TNBC




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download