1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015; 65:87–108. PMID:
25651787.

2. Rojas K, Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016; 59:651–672. PMID:
27681694.

3. Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture). Angew Chem Int Ed Engl. 2010; 49:7405–7421. PMID:
20821774.

4. Mathon NF, Lloyd AC. Cell senescence and cancer. Nat Rev Cancer. 2001; 1:203–213. PMID:
11902575.

5. Pfeiffer V, Lingner J. Replication of telomeres and the regulation of telomerase. Cold Spring Harb Perspect Biol. 2013; 5:a010405. PMID:
23543032.

6. Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci U S A. 1998; 95:90–92. PMID:
9419332.

7. Telomeres Mendelian Randomization Collaboration. Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, et al. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 2017; 3:636–651. PMID:
28241208.
8. Zhu X, Han W, Xue W, Zou Y, Xie C, Du J, et al. The association between telomere length and cancer risk in population studies. Sci Rep. 2016; 6:22243. PMID:
26915412.

9. Barwell J, Pangon L, Georgiou A, Docherty Z, Kesterton I, Ball J, et al. Is telomere length in peripheral blood lymphocytes correlated with cancer susceptibility or radiosensitivity? Br J Cancer. 2007; 97:1696–1700. PMID:
18000505.

10. De Vivo I, Prescott J, Wong JY, Kraft P, Hankinson SE, Hunter DJ. A prospective study of relative telomere length and postmenopausal breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2009; 18:1152–1156. PMID:
19293310.

11. Zheng YL, Ambrosone C, Byrne C, Davis W, Nesline M, McCann SE. Telomere length in blood cells and breast cancer risk: investigations in two case-control studies. Breast Cancer Res Treat. 2010; 120:769–775. PMID:
19543829.

12. Kim S, Sandler DP, Carswell G, De Roo LA, Parks CG, Cawthon R, et al. Telomere length in peripheral blood and breast cancer risk in a prospective case-cohort analysis: results from the Sister Study. Cancer Causes Control. 2011; 22:1061–1066. PMID:
21643930.

13. Svenson U, Nordfjäll K, Stegmayr B, Manjer J, Nilsson P, Tavelin B, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res. 2008; 68:3618–3623. PMID:
18483243.

14. Gramatges MM, Telli ML, Balise R, Ford JM. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer Epidemiol Biomarkers Prev. 2010; 19:605–613. PMID:
20142254.

15. Pellatt AJ, Wolff RK, Torres-Mejia G, John EM, Herrick JS, Lundgreen A, et al. Telomere length, telomere-related genes, and breast cancer risk: the breast cancer health disparities study. Genes Chromosomes Cancer. 2013; 52:595–609. PMID:
23629941.

16. Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, Santella RM. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007; 67:5538–5544. PMID:
17545637.

17. Shen J, Gammon MD, Terry MB, Wang Q, Bradshaw P, Teitelbaum SL, et al. Telomere length, oxidative damage, antioxidants and breast cancer risk. Int J Cancer. 2009; 124:1637–1643. PMID:
19089916.

18. Pooley KA, Sandhu MS, Tyrer J, Shah M, Driver KE, Luben RN, et al. Telomere length in prospective and retrospective cancer case-control studies. Cancer Res. 2010; 70:3170–3176. PMID:
20395204.

19. Qu S, Wen W, Shu XO, Chow WH, Xiang YB, Wu J, et al. Association of leukocyte telomere length with breast cancer risk: nested case-control findings from the Shanghai Women's Health Study. Am J Epidemiol. 2013; 177:617–624. PMID:
23444102.

20. Haiman CA, Chen GK, Vachon CM, Canzian F, Dunning A, Millikan RC, et al. A common variant at the TERT-CLPTM1L locus is associated with estrogen receptor-negative breast cancer. Nat Genet. 2011; 43:1210–1214. PMID:
22037553.
21. Palmer JR, Ruiz-Narvaez EA, Rotimi CN, Cupples LA, Cozier YC, Adams-Campbell LL, et al. Genetic susceptibility loci for subtypes of breast cancer in an African American population. Cancer Epidemiol Biomarkers Prev. 2013; 22:127–134. PMID:
23136140.

22. Bojesen SE, Pooley KA, Johnatty SE, Beesley J, Michailidou K, Tyrer JP, et al. Multiple independent variants at the TERT locus are associated with telomere length and risks of breast and ovarian cancer. Nat Genet. 2013; 45:371–384. PMID:
23535731.
23. Gao L, Thakur A, Liang Y, Zhang S, Wang T, Chen T, et al. Polymorphisms in the TERT gene are associated with lung cancer risk in the Chinese Han population. Eur J Cancer Prev. 2014; 23:497–501. PMID:
25254308.

24. Li G, Jin TB, Wei XB, He SM, Liang HJ, Yang HX, et al. Selected polymorphisms of GSTP1 and TERT were associated with glioma risk in Han Chinese. Cancer Epidemiol. 2012; 36:525–527. PMID:
22795327.

25. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002; 30:e47. PMID:
12000852.

26. Stewart SA, Weinberg RA. Telomeres: cancer to human aging. Annu Rev Cell Dev Biol. 2006; 22:531–557. PMID:
16824017.

27. Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016; 6:584–593. PMID:
27029895.

28. Shen J, Gammon MD, Terry MB, Bradshaw PT, Wang Q, Teitelbaum SL, et al. Genetic polymorphisms in telomere pathway genes, telomere length, and breast cancer survival. Breast Cancer Res Treat. 2012; 134:393–400. PMID:
22527105.

29. Rao S, Ye N, Hu H, Shen Y, Xu Q. Variants in TERT influencing telomere length are associated with paranoid schizophrenia risk. Am J Med Genet B Neuropsychiatr Genet. 2016; 171B:317–324. PMID:
26799699.
30. Pellatt AJ, Wolff RK, Lundgreen A, Cawthon R, Slattery ML. Genetic and lifestyle influence on telomere length and subsequent risk of colon cancer in a case control study. Int J Mol Epidemiol Genet. 2012; 3:184–194. PMID:
23050049.
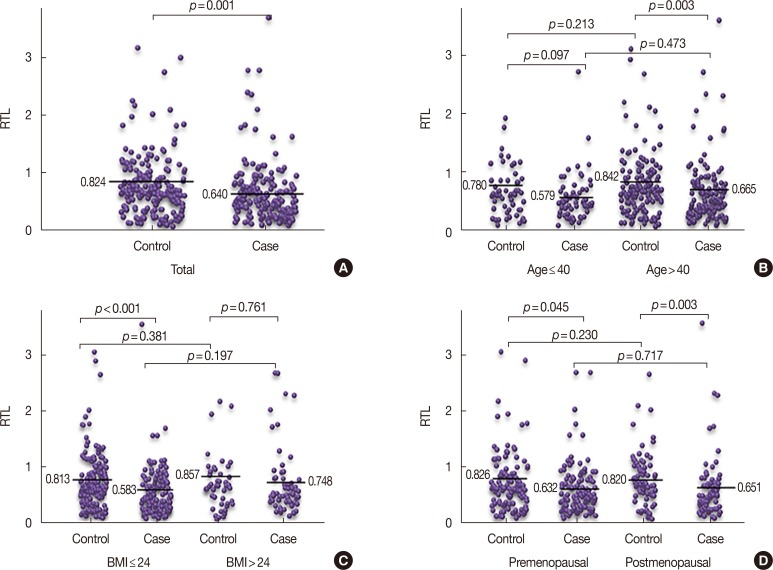
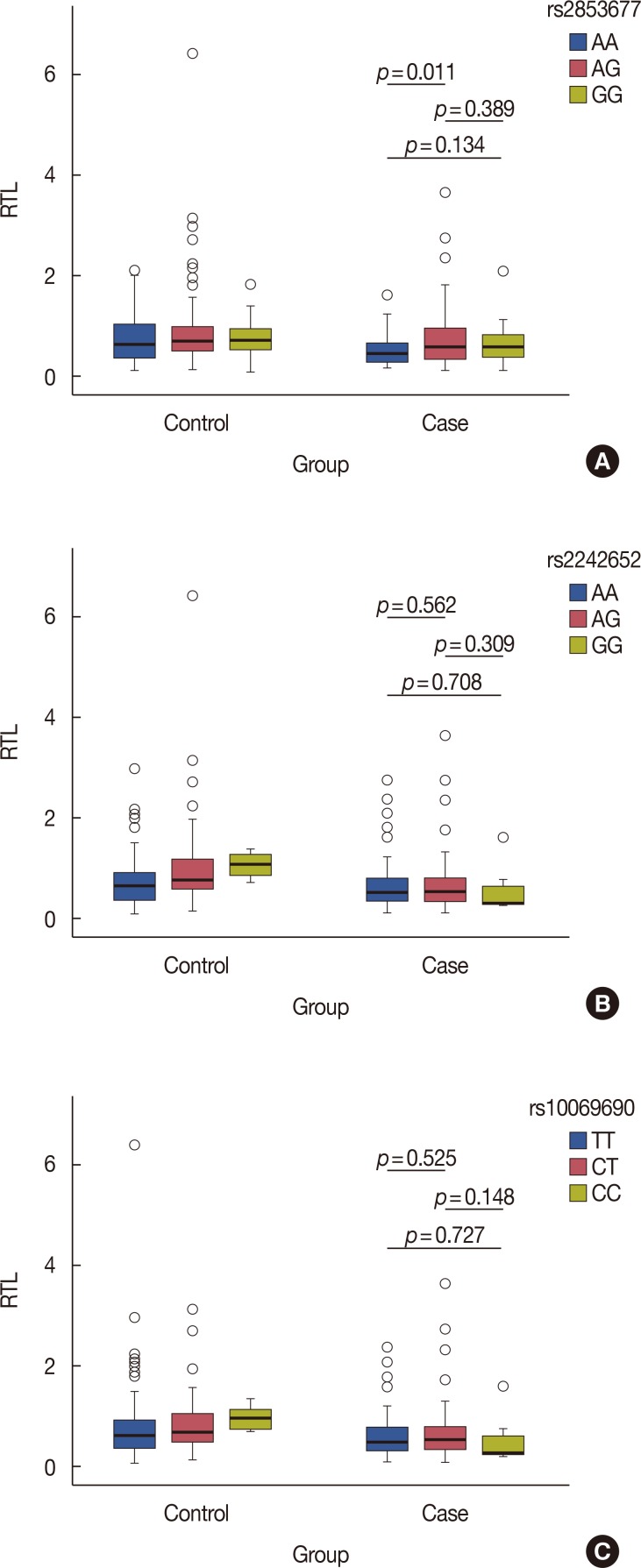




 PDF
PDF ePub
ePub Citation
Citation Print
Print



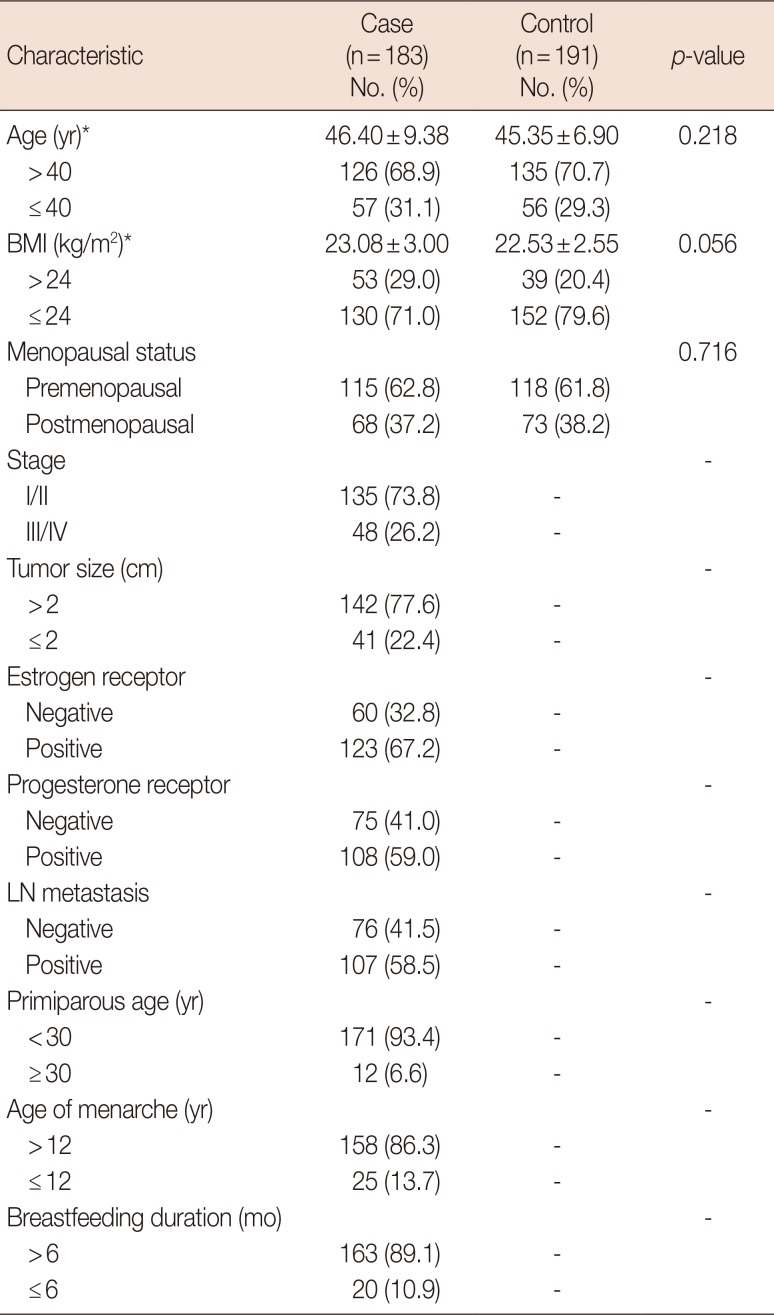
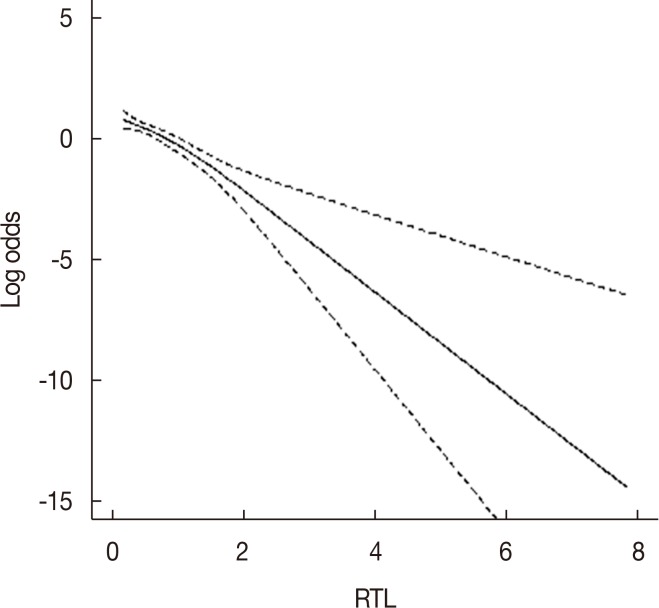
 XML Download
XML Download