This article has been
cited by other articles in ScienceCentral.
Abstract
In 2015, rapid human immunodeficiency virus (HIV) testing was implemented in all 25 public health centers in Seoul. During March and December 2015, 20,987 rapid HIV tests were performed, of which 116 (0.5%) were positive. Compared to those of the period before application of the rapid HIV test in place of conventional enzyme immunoassay method, the number of HIV tests performed and the number of positive results increased by sevenfold and twofold, respectively. In conclusion, expansion of the provision of rapid HIV tests in public health centers increased the number of voluntary HIV tests.
Keywords: Human immunodeficiency virus, Rapid HIV test, Public health center, Seoul, Korea
The epidemiology of human immunodeficiency virus (HIV) infection in Korea is characterized by an increasing number of newly diagnosed HIV cases and proportion of late-presentation diagnoses [
12]. Early diagnosis is important to decrease the opportunity for onward HIV transmission [
3], and voluntary HIV testing is considered an effective strategy to enhance early detection of HIV infection [
4]. Offering rapid HIV tests may encourage voluntary HIV testing [
56]. In this context, in 2014, use of rapid HIV test was encouraged in place of conventional enzyme immunoassay (EIA) method, anonymous HIV testing in four public health centers in Seoul [
7]. As a result of the implementing of rapid HIV tests, the average monthly numbers of HIV tests provided and HIV-positive diagnoses were approximately ninefold and sixfold greater, respectively, after adoption of the rapid HIV test. Based on these observations, rapid HIV testing was implemented in all 25 public health centers in Seoul in March 2015.
All of the 25 public health centers received additional funding to support their transition to rapid HIV testing, together with one or two additional laboratory technician(s). Rapid HIV testing and counseling are performed at isolated area for privacy of examinees. The public health center staff members were trained in this testing and discussion of the test results, and were educated about its advantages and disadvantages and the differences between rapid and conventional tests. Educative materials regarding rapid HIV testing for examinees were provided in the form of a brochure and booklet. To evaluate the frequency of HIV tests before and after adoption of rapid testing, the public health centers provided a monthly report on the total number of such tests performed, and their results.
The HIV testing algorithm was developed and revised after conducting a pilot project in 2014, and was included in the written protocol (
Fig. 1). Trained examiners offered the rapid test by finger-stick using the SD BIOLINE HIV-1/2 3.0 (Standard Diagnostics, Inc., Yongin-si, Korea) kit, which has high sensitivity and specificity, 100% and 99.3%, respectively [
8]. The EIA test was offered to examinees if they preferred it. Examinees were informed that the result of rapid HIV tests might be negative during the window period, which is longer than that of the fourth-generation EIA test, and they should be tested again if they might have been exposed to HIV recently. The results of the tests were confirmed by phone or by the staff member who performed the test 20 min after blood sampling. Examinees with positive test results were taken a further blood sample to confirm HIV infection by Western blot assay, performed by the Seoul Research Institute of Public Health and Environment or the Korea Centers for Disease Control and Prevention. The results were confirmed within 1 week by telephone, and those confirmed to have HIV infection were referred to staff for further counseling in the public health centers. This is a report of outcome of a public health strategy which have run by Seoul Metropolitan Government for public interest.
During March and December 2015, 20,987 rapid HIV tests were performed, of which 233 were positive; of these, 166 accepted confirmatory tests and 67 refused; 116 were confirmed to have HIV infection and 9 were indeterminate by Western blot. During 2012 and 2013, prior to adopting rapid HIV tests at the 25 district public health centers in Seoul, 4,267 and 3,654 conventional HIV tests were performed, of which 69 and 93 were positive (
Table 1). Compared to those in 2012 and 2013, the number of HIV tests performed and the number of positive results increased by sevenfold and twofold, respectively, during the study period.
Adoption of rapid HIV tests in public health centers was subject to several limitations. First, we could not determine the number of repeat tests in a single individual or whether known HIV-infected persons underwent rapid testing. Because rapid testing in public health centers is performed anonymously without personal information (as was the EIA test) to minimize the stigmatization issue. Thus, some examinees might have undergone multiple rapid tests. Second, the sensitivity of the rapid HIV test for detecting early-stage HIV infection is lower (22-33%
vs. 76-88%) than that of EIA tests [
9]. To minimize the problems due to the longer window period, examinees were provided information through educative materials and by trained staff members. In addition, the above information was offered to people who should be tested via the websites of public health centers and communities of men who have sex with other men. Anonymous HIV testing should be encouraged in groups at high risk for HIV infection [
10], thus, increasing the overall volume of voluntary HIV testing would benefit early detection of HIV infection and reduction of the risk of onward HIV transmission [
11].
In conclusion, expansion of the provision of rapid HIV tests in public health centers increased the number of voluntary HIV tests.
Figures and Tables
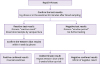 | Figure 1
Algorithm for use of rapid HIV tests in public health centers in Seoul. The EIA test was offered to examinees if they preferred. The processing of test results was identical for rapid and conventional testing.
HIV, human immunodeficiency virus; EIA, enzyme immunoassay.

|
Table 1
Frequency of performed HIV tests and confirmed HIV-positive cases by conventional EIA tests in 2012, 2013 and rapid HIV tests in 2015

|
2012 Conventional testsa
|
2013 Conventional testsa
|
2015 Rapid testsb
|
|
n (%) |
Monthly average |
n (%) |
Monthly average |
n (%) |
Monthly average |
|
Total |
4,267 (100) |
355.6 |
3,654 (100) |
304.5 |
20,987 (100) |
2,098.7 |
|
Confirmed HIV-positive |
69 (1.6) |
5.7 |
93 (2.5) |
7.7 |
116 (0.5) |
11.6 |

ACKNOWLEDGMENTS
The authors are grateful to the staff of the 25 public health centers in Seoul, and the anonymous reviewers whose comments significantly improved this paper.
References
1. Lee JH, Kim GJ, Choi BS, Hong KJ, Heo MK, Kim SS, Kee MK. Increasing late diagnosis in HIV infection in South Korea: 2000-2007. BMC Public Health. 2010; 10:411.

2. Kang CR, Bang JH, Cho SI, Kim KN, Lee HJ, Ryu BY, Cho SK, Lee YH, Oh MD, Lee JK. Patients presenting with advanced human immunodeficiency virus disease: epidemiological features by age group. J Korean Med Sci. 2016; 31:178–182.

3. Dennis AM, Napravnik S, Seña AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clin Infect Dis. 2011; 53:480–487.

4. Pilcher CD, Price MA, Hoffman IF, Galvin S, Martinson FE, Kazembe PN, Eron JJ, Miller WC, Fiscus SA, Cohen MS. Frequent detection of acute primary HIV infection in men in Malawi. AIDS. 2004; 18:517–524.

5. San Antonio-Gaddy M, Richardson-Moore A, Burstein GR, Newman DR, Branson BM, Birkhead GS. Rapid HIV antibody testing in the New York state anonymous HIV counseling and testing program: experience from the field. J Acquir Immune Defic Syndr. 2006; 43:446–450.
6. Gilbert M. Impact and use of point of care HIV testing: a public health evidence paper. BC Centre for Disease Control, STI/HIV. Prevention and Control. 2007.
7. Kang CR, Bang JH, Cho SI, Kim KN, Lee HJ, Lee YH, Ryu BY, Cho SK, Oh MD, Lee JK. Implementing the use of rapid HIV tests in public health centers in Seoul: results of a pilot project, 2014. J Korean Med Sci. 2016; 31:467–469.

8. World Health Organization (WHO). HIV assays: operational characteristics (phase 1). Report 14. Simple/rapid tests. Geneva, Switzerland: WHO;2004.
9. Patel P, Bennett B, Sullivan T, Parker MM, Heffelfinger JD, Sullivan PS. CDC AHI Study Group. Rapid HIV screening: missed opportunities for HIV diagnosis and prevention. J Clin Virol. 2012; 54:42–47.

10. Mimiaga MJ, Reisner SL, Bland S, Skeer M, Cranston K, Isenberg D, Vega BA, Mayer KH. Health system and personal barriers resulting in decreased utilization of HIV and STD testing services among at-risk black men who have sex with men in Massachusetts. AIDS Patient Care and STDS. 2009; 23:825–835.

11. Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005; 39:446–453.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download