Abbreviations
allo
APC
B6
BLI
BM
BMT
BrdU
cDC
DC
FDR
GMFI
GSEA
GVHD
iNOS
IRF
KO
Ly6G+
MACS
MDSC
MiHA
MST
MyD88
PBL
PMN
RT
TCD
TipDC
TRIF
VSV
WT
INTRODUCTION
MATERIALS AND METHODS
Mice
Induction of acute GVHD and bioluminescence imaging (BLI) analysis
Flow cytometric analysis
Microarray analysis
Immune suppression and Ag-presentation assays
In vivo bromodeoxyuridine (BrdU) assay
Bioinformatic analysis of the gene expression profiles
RESULTS
Enhanced T cell alloreactivity in B6→BALB.B GVHD hosts with MyD88-KO BMT
 | Figure 1Aggravated GVHD in the recipients of MyD88-KO BM with allo WT T cells. (A) A schematic diagram of the experimental GVHD mouse model. WT (or KO) indicates MyD88 WT (or MyD88 KO) of B6 mice, T indicates WT type splenic T cells of B6 mice, BM indicates TCD BM; syn WT (or KO) BM+WT T (B6→B6); allo WT (or KO)+WT T (B6→BALB.B); allo WT (or KO) BM (BM only). (B) Survival, GVHD score, and weight loss in acute GVHD mice (n=10 per group). Data (B) represent 3 independent experiments. |
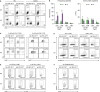 | Figure 2T cells originated from MyD88 KO BM GVHD hosts were less apoptotic, more proliferative, and more potent in effector functions. (A, B) The proportion and numbers of donor CD4+ and CD8+ T cells in PBL and spleen were determined on day 7 post-transplantation. (C) BrdU incorporation assays for proliferation of donor-derived T cells in spleen from the indicated GVHD groups on day 5 and 7 post-transplantation. (D) Annexin V/DAPI staining for detection of apoptosis of donor-derived T cell subsets in spleen from the indicated GVHD groups on day 7 post-transplantation. (E) Intracellular IFN-γ- and (F) GZMB staining in donor-derived T cells in spleen from the indicated GVHD groups on day 7 post-transplantation. Data are expressed as the % mean± standard deviation. Data (A-F) represent 3 independent experiments.
*p<0.05; **p<0.01; ***p<0.001 (WT vs. KO).
|
Distinct in vivo dynamics of MyD88-KO BM progenies in GVHD hosts
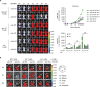 | Figure 3In vivo dynamics of MyD88 KO BM and WT BM progenies in different conditions of recipients. (A) BLI analysis for in vivo dynamics of TCD-BM (non-T cell compartments) cell expansion after transplantation. The migration and expansion of TCD-BM cells were longitudinally monitored at 2 h, days 1, 3, 5, 7, 8 and 9 post-transplantation using an IVIS imaging system 100 (Xenogen, Alameda, CA, USA). (B) At days 5, 7, and 9 post-transplantation, individual target organs, Sp (spleen), Li (liver), Lu (lungs), Ki (kidney), and Int (intestines), were surgically removed from recipient mice and measured for donor-originated infiltrating TCD-BM cells. Representative imaging data are shown. Total body flux (photons/s) values were measured and plotted using Living Image Software (Xenogen) and statistical significance of total body flux at days 5, 7, and 9 was determined. Data (A, B) represent 3 independent experiments. |
Enhanced apoptosis of CD11b+Gr-1+ cells in MyD88-KO GVHD hosts
 | Figure 4Flow cytometric analysis of CD11b+Gr-1+ cells in progenies in different conditions of BM recipients. (A) Proportion of donor CD11b+ and CD11b+Gr-1+ cells in GVHD and non-GVHD hosts after excluding T cell proportions in spleen on days 5, 7, and 9 post-transplantation. (B) The frequencies and absolute numbers of donor-originated total CD11b+ cell and (C) CD11b+Gr-1+ cells in spleen on days 5, 7, and 9 post-transplantation. (D) Proportion of donor CD11b+Gr-1+ cells after excluding T cell proportions (CD3-β2mb+) in BM on days 5, 7, and 9 post-transplantation, including intestine, on day 9 post-transplantation. (E) Frequencies and absolute numbers of donor-originated CD11b+Gr-1+ cells in BM on days 5, 7, and 9 post-transplantation and intestine on day 9 post-transplantation. (F) Representative frequencies of Ly6GhiLy6Clow or Ly6GlowLy6Chi subpopulations within the donor-originated (β2mb+) CD11b+ cells in spleen on days 5, 7, and 9 post-transplantation. (G) Frequencies and absolute numbers of Ly6GhiLy6Clow or Ly6GlowLy6Chi subpopulations within the donor-originated (β2mb+) CD11b+ cells post-transplantation. (H) Apoptosis of donor-derived CD11b+Gr-1+ cells in GVHD host was analyzed by staining with annexin V Ab on days 5 and 7 post-transplantation. The GMFI values were measured and plotted. Data (A-H) represent more than 3 independent experiments.
*p<0.05; **p<0.01; ***p<0.001 (WT vs. KO).
|
Apoptosis- and DC differentiation-prone gene expression by CD11b+Gr-1+ cells from MyD88-KO GVHD hosts
 | Figure 5Differential gene expression profiles between splenic MyD88 KO and WT CD11b+Gr-1+ cells on day 7 post-transplantation in GVHD hosts. (A) Representative proportion of donor-originated CD11b+Gr-1+ cells in spleen on day 7 post-transplantation. Gr-1+ cells in GVHD hosts were all CD11b+ cells. (B) Heat map for relative gene expression in CD11b+Gr-1+ cells between WT and MyD88 KO GVHD hosts. (C) Enrichment of genes for inflammatory response by GSEA with selected gene lists. The FDR q<0.04 indicates specific enrichment of the gene set. Leading edge subset analysis indicates that genes are highly correlated with the inflammatory response. (D) Venn diagram of differentially expressed gene profiles between splenic CD11b+Gr-1+ cells from MyD88 and WT GVHD hosts by the indicated pair comparisons. Each number indicates the number of up- and downregulated unique genes, or shared genes. (E) The top 5 significant biological categories modulated by absence of MyD88 in CD11b+Gr-1+ cells in GVHD hosts. (F) Networks of genes in apoptosis of leukocyte and cytolysis related genes regulated by MyD88-deficiency in GVHD development. (G) Heat map for differential expression of Ag-presentation-related genes, cytokines, chemokines, metabolism, signaling, and transcription in splenic CD11b+Gr-1+ cells between WT and MyD88 KO GVHD hosts on day 7 post-transplantation. |
Enhanced DC fractions in CD11b+Gr-1+ cells of MyD88-KO GVHD hosts
 | Figure 6Increased proportion of DCs in MyD88-KO CD11b+Gr-1+ cells from GVHD hosts. (A, B) The composition of CD11b+Gr-1+ cells from spleen of allo GVHD hosts on day 7 post-transplantation. PMN-MDSCs (CD11c−CD11b+CD244+Ly6GhiLy6Cint) and M-MDSCs (CD11c−CD11b+CD124+Ly6GlowLy6Chi) including neutrophils (CD11c−CD11b+CD244−Ly6GhiLy6Cint) and monocytes (CD11c−CD11b+CD124−Ly6GlowLy6Chi). (C) Flow cytometric analysis of MHC class II, CD80, and CD86 on CD11c+CD11b+Gr-1+ cells isolated from spleen of WT and MyD88 KO BM GVHD hosts on day 7 post-transplantation. The Geom. MFI was measured and plotted. (D) Proportion of annexin V+ cells in CD11b+Gr-1+CD11c+ cells from spleen of allo GVHD hosts on day 7 post-transplantation. (E) Ag-presentation assay. Equal numbers of CFSE-labeled J15 CD8+ T cells (CD45.1+) were co-cultured with Gr-1+ cells (1×105 cells) isolated from spleen in WT or MyD88 KO BM GVHD hosts on day 7 post-transplantation, in the presence of H60 peptide (1 µM) or VSV control peptide (1 µM) for 3 days. The proliferation of J15 CD8+ T cells (CD45.1+) elicited by H60 peptide was measured by CFSE dilution on day 3 by flow cytometry, and the proportion of proliferation is presented as mean±standard deviation. (F) Immunosuppression assay using Gr-1+ cells isolated from spleen in WT or MyD88-KO BM allo GVHD hosts on day 7 post-transplantation. CFSE-labeled CD45.1+ T cells (1×105) were co-cultured with equal numbers of Gr-1+ cells. T cells only or mixed cells (1:1) were incubated in the absence or presence of anti-CD3 (2 µg/ml)/CD28 (1 µg/ml). The proliferation of CD45.1+ T cells was measured by CFSE dilution after 3 days by flow cytometry. The proportions of proliferated CD45.1+ CD4+ and CD45.1+ CD8+ T cells were indicated and plotted. Data (B-F) represent 3 independent experiments.
*p<0.05; **p<0.01; ***p<0.001 (WT vs. KO).
|
Preferential DC differentiation of MyD88-KO BM cells under in vitro MDSC producing conditions
 | Figure 7Flow cytometric analysis of BM progenies generated by in vitro culture using MyD88 KO BM and WT BM donor mice. (A) Number of total cells after 4 days cultivation of TCD-BM of MyD88 KO and WT donor mice with GM-CSF plus LPS. (B) Proportion of annexin V+ cells in in vitro cultured TCD-BM of MyD88 KO and WT donor mice for 4 days with GM-CSF (40 ng/ml) plus LPS (1 µg/ml), (C) the proportion of CD11b+Gr-1+ MDSCs from TCD-BM of MyD88 KO and WT donor mice, and number of total cells after 4 days cultivation with GM-CSF plus LPS. PMN-MDSCs (CD11c−CD11b+Ly6GhiLy6ClowCD244+), and M-MDSCs (CD11c−CD11b+Ly6GlowLy6ChiCD124+), DC (CD11c+), neutrophils (CD11c−CD11b+Ly6GhiLy6ClowCD244−), and monocytes (CD11c−CD11b+Ly6GlowLy6ChiCD124−). (D) Flow cytometry analysis showing decreased expression of Arg-1 and iNOS on day 4 within PMN-MDSCs and M-MDSCs gated subpopulations in in vitro cultured TCD-BM cells from WT and MyD88-KO donor mice. The Geom. MFI was measured and plotted. Data (A-D) represent 3 independent experiments.
*p<0.05 (WT vs. KO).
|
 | Figure 8Functional tests of CD11b+Gr-1+ cells generated by in vitro culture using MyD88 KO BM and WT BM donor mice. (A) In vitro cultured-TCD-BM cells were co-cultured at a 1:1 ratio with CFSE-labeled T cells (1×105) and stimulated with anti-CD3/CD28 antibodies for 3 days. The proliferation of J15 CD8+ T cells (CD45.1+) was determined by flow cytometry. (B) Ag-presentation assay was performed as described in Fig. 6E. CFSE-labeled J15 CD8+ T cells (CD45.1+) were co-cultured with WT or MyD88 KO TCD-BM (1×105 cells) cultured in vitro in the presence of GM-CSF plus LPS for 4 days. After co-cultivation in the presence of H60 peptide (1 µM) or VSV control peptide (1 µM) for 3 days, the proliferation of J15 CD8+ T cells (CD45.1+) was determined by flow cytometry. Data (A and B) represent 3 independent experiments. |
Cross-presentation of alloantigens and death of antigen presenting cells (APCs) in Gr-1+ cells of MyD88-KO GVHD hosts
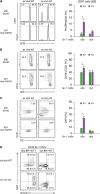 | Figure 9MyD88 KO BM-derived DCs isolated from GVHD hosts are able to cross-present Allo-Ag. (A) Ag-presentation assay was performed using WT polyclonal CD8+ T cells. CFSE-labeled WT B6 CD8+ T cells (CD45.1+) were co-cultured with Gr-1+ cells (1×105 cells) isolated from spleen of WT and MyD88 KO allogenic GVHD hosts including syngeneic non-GVHD control mice. After co-cultivation for 3 days, the proliferation of polyclonal WT CD8+ T cells (CD45.1+) was plotted. (B) The CD44 expression on CD8+ T cells was measured by flow cytometry. (C) The proportion of DAPI+ cells among non-T cell compartments (Gr-1+ cells) was plotted. (D) Apoptosis of CD11b+CD11c+ cells from WT and MyD88-KO BM GVHD (allo), and non-GVHD hosts (syn) was analyzed by following annexin V staining on day 7 post-transplantation. Data (A-D) represent 2 independent experiments.
*p<0.05 (WT vs. KO).
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download