1. Dixon J, Lane K, Macphee I, Philips B. Xenobiotic metabolism: the effect of acute kidney injury on non-renal drug clearance and hepatic drug metabolism. Int J Mol Sci. 2014; 15(2):2538–2553.

2. Philips BJ, Lane K, Dixon J, Macphee I. The effects of acute renal failure on drug metabolism. Expert Opin Drug Metab Toxicol. 2014; 10(1):11–23.

3. Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014; 25(9):1897–1907.

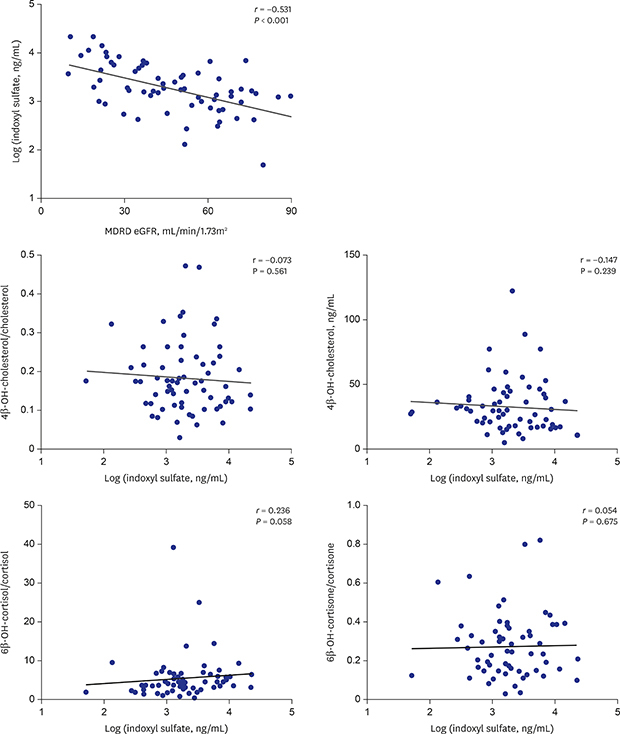
4. Suzuki Y, Itoh H, Fujioka T, Sato F, Kawasaki K, Sato Y, et al. Association of plasma concentration of 4β-hydroxycholesterol with CYP3A5 polymorphism and plasma concentration of indoxyl sulfate in stable kidney transplant recipients. Drug Metab Dispos. 2014; 42(1):105–110.

5. Hanada K, Ogawa R, Son K, Sasaki Y, Kikkawa A, Ichihara S, et al. Effects of indoxylsulfate on the in vitro hepatic metabolism of various compounds using human liver microsomes and hepatocytes. Nephron, Physiol. 2006; 103(4):179–186.
6. Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T. Tissue distribution of mRNA expression of human cytochrome P450 isoforms assessed by high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi. 2003; 123(5):369–375.
7. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013; 138(1):103–141.

8. Knights KM, Rowland A, Miners JO. Renal drug metabolism in humans: the potential for drug-endobiotic interactions involving cytochrome P450 (CYP) and UDP-glucuronosyltransferase (UGT). Br J Clin Pharmacol. 2013; 76(4):587–602.

9. Michaud J, Nolin TD, Naud J, Dani M, Lafrance JP, Leblond FA, et al. Effect of hemodialysis on hepatic cytochrome P450 functional expression. J Pharmacol Sci. 2008; 108(2):157–163.

10. Oh J, Kim AH, Lee S, Cho H, Kim YS, Bahng MY, et al. Effects of renal impairment on the pharmacokinetics and pharmacodynamics of a novel dipeptidyl peptidase-4 inhibitor, evogliptin (DA-1229). Diabetes Obes Metab. 2017; 19(2):294–298.

11. Yeung CK, Shen DD, Thummel KE, Himmelfarb J. Effects of chronic kidney disease and uremia on hepatic drug metabolism and transport. Kidney Int. 2014; 85(3):522–528.

12. Doogue MP, Polasek TM. Drug dosing in renal disease. Clin Biochem Rev. 2011; 32(2):69–73.
13. Monteiro MS, Carvalho M, Bastos ML, Guedes de Pinho P. Metabolomics analysis for biomarker discovery: advances and challenges. Curr Med Chem. 2013; 20(2):257–271.

14. Watanabe M, Kumai T, Matsumoto N, Tanaka M, Suzuki S, Satoh T, et al. Expression of CYP3A4 mRNA is correlated with CYP3A4 protein level and metabolic activity in human liver. J Pharmacol Sci. 2004; 94(4):459–462.

15. Liu CH, Peck K, Huang JD, Lin MS, Wang CH, Hsu WP, et al. Screening CYP3A single nucleotide polymorphisms in a Han Chinese population with a genotyping chip. Pharmacogenomics. 2005; 6(7):731–747.

16. Shin KH, Choi MH, Lim KS, Yu KS, Jang IJ, Cho JY. Evaluation of endogenous metabolic markers of hepatic CYP3A activity using metabolic profiling and midazolam clearance. Clin Pharmacol Ther. 2013; 94(5):601–609.

17. Kim B, Moon JY, Choi MH, Yang HH, Lee S, Lim KS, et al. Global metabolomics and targeted steroid profiling reveal that rifampin, a strong human PXR activator, alters endogenous urinary steroid markers. J Proteome Res. 2013; 12(3):1359–1368.

18. Lee J, Kim AH, Yi S, Lee S, Yoon SH, Yu KS, et al. Distribution of exogenous and endogenous CYP3A markers and related factors in healthy males and females. AAPS J. 2017; 19(4):1196–1204.

19. Moon JY, Kang SM, Lee J, Cho JY, Moon MH, Jang IJ, et al. GC-MS-based quantitative signatures of cytochrome P450-mediated steroid oxidation induced by rifampicin. Ther Drug Monit. 2013; 35(4):473–484.

20. de Graan AJ, Sparreboom A, de Bruijn P, de Jonge E, van der Holt B, Wiemer EA, et al. 4β-Hydroxycholesterol as an endogenous CYP3A marker in cancer patients treated with taxanes. Br J Clin Pharmacol. 2015; 80(3):560–568.

21. Bodin K, Andersson U, Rystedt E, Ellis E, Norlin M, Pikuleva I, et al. Metabolism of 4 beta-hydroxycholesterol in humans. J Biol Chem. 2002; 277(35):31534–31540.
22. Diczfalusy U, Nylén H, Elander P, Bertilsson L. 4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol. 2011; 71(2):183–189.

23. Mårde Arrhén Y, Nylén H, Lövgren-Sandblom A, Kanebratt KP, Wide K, Diczfalusy U. A comparison of 4β-hydroxycholesterol : cholesterol and 6β-hydroxycortisol: cortisol as markers of CYP3A4 induction. Br J Clin Pharmacol. 2013; 75(6):1536–1540.
24. Diczfalusy U, Kanebratt KP, Bredberg E, Andersson TB, Böttiger Y, Bertilsson L. 4beta-hydroxycholesterol as an endogenous marker for CYP3A4/5 activity. Stability and half-life of elimination after induction with rifampicin. Br J Clin Pharmacol. 2009; 67(1):38–43.
25. Furuta T, Suzuki A, Mori C, Shibasaki H, Yokokawa A, Kasuya Y. Evidence for the validity of cortisol 6 beta-hydroxylation clearance as a new index for in vivo cytochrome P450 3A phenotyping in humans. Drug Metab Dispos. 2003; 31(11):1283–1287.
26. Chan KC, Lit LC, Law EL, Tai MH, Yung CU, Chan MH, et al. Diminished urinary free cortisol excretion in patients with moderate and severe renal impairment. Clin Chem. 2004; 50(4):757–759.

27. Kim AH, Kim B, Rhee SJ, Lee Y, Park JS, Lee SM, et al. Assessment of induced CYP3A activity in pregnant women using 4β-hydroxycholesterol: cholesterol ratio as an appropriate metabolic marker. Drug Metab Pharmacokinet. 2018; 33(3):173–178.








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download