Abstract
Secondary hyperparathyroidism (SHPTH) occurs commonly in patients with end-stage renal disease (ESRD). Uncontrolled SHPTH is associated with complications of calcium deposition including calciphylaxis and elevated rates of cardiovascular morbidity. Current treatment recommendations for medically refractory disease include total parathyroidectomy, often with autotransplantation (TPTH+AT) of minced parathyroid gland. Surgical intervention is associated with a reduction in cardiovascular mortality. We report a case of a 56-year-old man with ESRD who developed SHPTH and underwent TPTH+AT of parathyroid tissue into the right brachioradialis muscle. Over the course of 7 years he developed a mass at the site of the autotransplanted gland as well as recurrent refractory hyperparathyroidism with increased forearm uptake noted on sestamibi scan. After excision of this mass, pathology demonstrated hyperplasia of the minced gland fragments which were embedded within a mass of fibroadipose tissue rather than the muscle tissue it was originally transplanted in.
Secondary hyperparathyroidism (SHPTH) is a common malady experienced by patients with end-stage renal disease (ESRD) and has a reported incidence of between 30%–50% in this population (1). Complications of excessive parathyroid hormone (PTH) can include interstitial and vascular complications, including cardiovascular morbidity and mortality (2). Resection of medically refractory SHPTH has been associated with improved mortality and cardiovascular related mortality (34). As such, treatment of SHPTH in patients with ESRD refractory to medical therapy has been subtotal or total parathyroidectomy (TPTX) with or without autotransplantation. Rising PTH levels after TPTX raises concern for hyperplasia of any native remnant parathyroid or of the autotransplanted parathyroid tissue. We report a case of a man who presented with PTH >3,000 pg/mL 7 years after TPTX with right forearm autotransplantation.
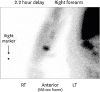
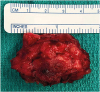
This is a 56-year-old man with a history of a stroke, paroxysmal atrial fibrillation and ESRD due to hypertension. He had developed SHPTH which was treated over a two-year period but was ultimately refractory to medical management. He then underwent TPTX with right forearm autotransplantation of several 1×1×1 mm pieces of parathyroid gland into the right brachioradialis muscle in April 2011. The fragments were implanted though the fascia into the muscle via several small adjacent incisions with closure of the fascia after transplantation. He had been followed by his nephrologist and was noted to have a rising PTH in October 2014 and it remained elevated between 3,000 and 3,500 pg/mL. During this period of time, he also noted gradual growth of a right forearm mass under the prior incision. In November 2017, he developed bilateral lower extremity ulcers due to calciphylaxis and was admitted to the hospital in April 2018 with concern for underlying soft tissue infection of these lower extremity wounds. At the time of admission, the right forearm mass was noted to be 5×4.1 cm and located directly under his previous surgical scar. The mass was soft and slightly fixed to the underlying tissue. On admission, his serum calcium level was 8.2 mg/dL and his PTH was 3,242 pg/mL. A bedside evaluation for the presence of functional parathyroid tissue in the right forearm was achieved by applying a tourniquet to the proximal right arm for 20 minutes. Repeated PTH level had decreased to 2,069 pg/mL, approximately 2/3 the pretest value. Computed tomography (CT) imaging demonstrated a 5.7 × 4.3 cm right forearm soft tissue mass. A sestamibi scan demonstrated uptake in the right forearm and neck (Fig. 1). He then underwent resection of the right forearm mass (Fig. 2). A serum PTH was drawn 20 minutes after resection and was noted to be 2,274 pg/mL. Pathology demonstrated multiple hyperplastic parathyroid nodules randomly distributed within the right forearm fibroadipose mass, ranging in size from 0.5 to 1.6 cm in diameter. Repeated sestamibi did not demonstrate uptake in the right forearm however a 4D CT scan of the neck demonstrated likely remnant hyperplastic parathyroid nodule in the anterior left neck and he was referred to otolaryngology for repeated resection. He subsequently underwent exploration and resection of hyperplastic remnant parathyroid tissue in July 2018 resulting in normalization of his PTH levels.
SHPTH continues to be treated surgically with either subtotal or TPTX with autotransplantation (TPTX+AT) of sectioned parathyroid tissue. Even after resection, SHPTH may recur and continue to contribute to poor outcomes including calciphylaxis and advanced vascular disease. Small scale studies have reported mixed conclusions regarding which approach is superior, although for some patients, there may be specific advantages favoring one approach over the other. Li (4) reported on 62 patients who underwent TPTX compared to 42 patients who underwent TPTX+AT. Twelve months after surgery, they observed improved rates of coronary artery calcification in those treated with TPTX+AT vs. TPTX as well as lower recurrence rate of SHPT at 12 months. In terms of safety, either approach appears to offer similar outcomes. In 2017, Anderson (3) reported on 1,130 patients from data extracted from the NSQIP ACS database; 365 patients underwent TPTX+AT and the remainder underwent subtotal parathyroidectomy. There were no significant differences in complication rate, 30-day mortality rate or 30-day readmission. Long term follow-up in this population with regard to recurrence of SHPT has also been documented. The study by Tominaga (5) in 2010, evaluated 2,260 patients with ESRD who underwent TPTX with forearm autotransplantation over a 29-year period. They reported a rising cumulative frequency of autograft removal for treatment of recurrent SHPTH; 7.5% of patients required removal at 5 years, 17.4% at 10 years and 25.8% at 20 years. Removal of the autotransplanted gland via en bloc resection of the muscle was required in 248 patients and reoperation to remove remnant parathyroid tissue in the forearm was required in 45 patients. Interestingly, 18 patients required additional cervical or thymic resection to remove residual adenomas.
In our report, we observed the presence of multiple large hyperplastic parathyroid nodules randomly distributed within a large mass of fibroadipose tissue after surgical implantation into the brachioradialis muscle. The rich vascular supply of muscle tissue is likely required, at least initially, to support viability of the 1 mm parathyroid tissue fragments and according to longitudinal studies, autotransplanted parathyroid gland appears to remain within the muscle. In this case, migration and hypertrophy of the transplanted glandular tissue over a period of years into the fatty stroma was observed.
Liu et al. (2) commented that ongoing hemodialysis and specific environmental factors, including altered calcium and phosphorous regulation, elevated Fibroblast Growth Factor-23 and other factors may lead to persistent stimulation of autotransplanted parathyroid graft tissue and result in cell proliferation and excess secretion of PTH. This mechanism may account for the hyperplasia noted in the resected specimen in this case.
Co-stimulation between various signaling pathways within the microenvironment encountered by the transplanted glandular tissue and surrounding muscle and adipose is possible but whether or not this can influence growth or tissue migration remains speculative. In our patient, complete resection and the absence of forearm enhancement on the post resection sestamibi scan, demonstrated the absence of residual glandular tissue within the muscular compartment suggesting complete migration of all the previously autotransplanted parathyroid tissue. No clear and scientific explanation for the observed migration into fatty stroma has been reported.
SHPT is common in patients with ESRD and can lead to significant morbidity. Cases of medically refractory SHPT continue to be treated surgically. Recurrence of SHPT is also common and may require resection of the remnant native parathyroid tissue or the autotransplanted parathyroid tissue, or both. Migration and proliferation within fatty stroma of transplanted parathyroid tissue is a unique occurrence and may be underreported. Additional detailed molecular analysis may further explain this phenomenon.
Notes
Author Contributions
Conceptualization: Joseph Arturo Reza.
Data curation: Joseph Arturo Reza.
Methodology: Joseph Arturo Reza.
Project administration: Joseph Dominic Portoghese.
Supervision: Joseph Dominic Portoghese.
Writing - original draft: Joseph Arturo Reza.
Writing - review & editing: Georg Kristof Wiese, Joseph Dominic Portoghese.
References
1. Hedgeman E, Lipworth L, Lowe K, Saran R, Do T, Fryzek J. International burden of chronic kidney disease and secondary hyperparathyroidism: a systematic review of the literature and available data. Int J Nephrol. 2015; 2015:184321.

2. Liu ME, Qiu NC, Zha SL, Du ZP, Wang YF, Wang Q, et al. To assess the effects of parathyroidectomy (TPTX versus TPTX+AT) for secondary hyperparathyroidism in chronic renal failure: a systematic review and meta-analysis. Int J Surg. 2017; 44:353–362.

3. Anderson K Jr, Ruel E, Adam MA, Thomas S, Youngwirth L, Stang MT, et al. Subtotal vs. total parathyroidectomy with autotransplantation for patients with renal hyperparathyroidism have similar outcomes. Am J Surg. 2017; 214:914–919.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download