INTRODUCTION
Lymphatic malformations, previously called lymphangiomas, are malformations of the lymphatic system, consisting of cystic, dilated lymphatic vessels [
12]. These malformations usually occur in children younger than 2 years of age and are common in the neck, head and armpit, but can also occur in the spleen, liver, mesentery, omentum and retroperitoneum [
34567891011]. Lymphatic malformations in the abdomen are rarely found in children and may present as asymptomatic abdominal masses. However, emergency surgery is required because of complications such as abdominal pain, high fever, intestinal obstruction, infection, hemorrhage, and torsion [
12131415]. The treatment of choice is complete resection, as incomplete resection is associated with a high incidence of relapse [
1415]. This study reviewed the clinical features, diagnosis, and treatment outcomes of pediatric patients treated at our institution for lymphatic malformations of the abdomen.
DISCUSSION
Lymphatic malformations are cystic tumors that develop slowly and are due to the failure of communication between lymphatic vessels during the fetal period. Lymphatic malformations can be differentiated from mesenteric cysts arising from mesothelial tissue. Lymphatic malformations are pathologically diagnosed by the presence of squamous epithelium, a small lymphatic space, abundant lymphoid tissue, smooth muscle in the walls of the cyst, and foam cells containing lipid material [
46]. About 95% of these malformations occur in the neck and axilla, with fewer than 5% occurring in other parts of the body, including the abdomen. Symptoms of lymphatic malformations occur in 50%–60% of patients before age 1 year and in 90% before age 2 years [
29].
Although the incidence of abdominal lymphatic malformation is not known precisely, they account for 3%–9.2% of lymphatic malformations in children [
2]. Moreover, fewer than 1% of lymphatic malformations in the abdomen occur in the retroperitoneum. Abdominal lymphatic malformations occur most frequently in the mesentery, followed by the greater omentum and retroperitoneum. Mesenteric lymphatic malformations can occur in any part of the mesentery, from the duodenum to the rectum, but are most prevalent in the small intestine, especially in the ileum. However, 1 study reported that malformations were most prevalent in the jejunum (44%), and that colon malformations were most common in the transverse mesocolon [
1]. Of our 12 patients, 4 had lymphatic malformations in the retroperitoneum, 4 in the greater omentum, 2 in the jejunal mesentery, and 2 in the retroperitoneum and mesentery.
Previous studies have reported that abdominal lymphatic malformations tend to occur mostly in males, with a male to female ratio of 1.5:1 to 3.2:1 [
414]. Our findings are in good agreement, showing a male to female ratio of 11:1.
Abdominal lymphatic malformation can present with various symptoms, including abdominal pain, abdominal distension, abdominal mass, nausea, vomiting, diarrhea, and constipation, depending on the location and size of the lesion. These malformations, however, can also be diagnosed by the occurrence of intestinal obstruction due to torsion or extrinsic compression, as well as by complications such as bleeding into the cysts and peritonitis due to infection or perforation [
1617]. Clinical features differ in children and adults. In adults, the symptoms are usually mild, nonspecific, and chronic, with a relatively long duration prior to diagnosis. Because the abdominal cavity is smaller in children than in adults, the duration of symptoms is shorter, and symptoms are more acute [
2418].
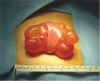
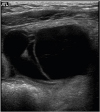
Abdominal lymphatic malformations are very difficult to diagnose before surgery, with most patients diagnosed by postoperative biopsy. No specific radiologic findings can confirm abdominal lymphatic malformations before surgery. Abdominal ultrasonography or CT is necessary for accurate diagnosis before surgery, with these modalities providing information on lesion location and size and association with adjacent lesions. On abdominal ultrasonography, these lesions appear as hypoechoic cystic masses with a round wall separated by echogenic septa in the cystic space [
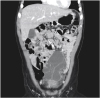
37]. Abdominal CT scans provide more information before surgery about lesion location, size, and association with adjacent lesions. Typically, shading of the contents is homogeneous, with CT scans showing a single or polycystic mass with thin walls, as well as showing the relationship of the lesion with adjacent organs and blood vessels [
319]. Abdominal magnetic resonance imaging (MRI) does not provide more information than ultrasonography or CT about lesion location, but it may be helpful in diagnosing complications such as hemorrhage. CT scanning is useful diagnostically, but ultrasonography and MRI may be better for children because of low radiation exposure. The differential diagnosis of abdominal lymphatic malformations includes other cystic abdominal masses, such as choledochal cysts, urachal cysts, ovarian cysts, renal cysts, intestinal duplications, abdominal lymphoma, simple mesenteric cysts, and pancreatic pseudocysts [
82021].
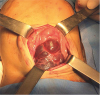
Treatment of abdominal lymphatic malformation consists of complete surgical resection, as the outcome of other treatments, such as aspiration, drainage, and irradiation, are poor [
4]. Once diagnosed, lesion size and the frequency of complications, such as infection, hemorrhage, and torsion, increase over time, suggesting the need for early surgical treatment. The timing of surgery should be determined in relation to the symptoms. Emergency surgery is required for patients with complications, such as intestinal obstruction, torsion, and rupture, whereas conservative treatment may be sufficient for patients with peritonitis due to infection or hemorrhage, followed by surgery after the symptoms have improved [
2]. Lymphatic malformations in the mesentery and greater omentum are more likely to require emergency surgery because they are more likely to be associated with complications than lymphatic malformations in the retroperitoneum. The location and size of lymphatic malformations of the mesentery, as well as their relationships with adjacent organs, may affect the outcomes of resection. Pathologic findings of lymphatic malformation may affect the surgical technique and the possibility of complete resection. Complete resection of macrocystic type malformations is easy because of their clear boundaries. However, complete resection of microcystic or mixed type lesions accompanied by extensive invasion of other organs is more difficult because the boundaries are unclear. Lymphatic malformations of the mesentery have been classified into 4 groups based on surgical findings, with this classification helping to choose the treatment or surgical method [
1]. Abdominal lymphatic malformations, although benign, may invade locally, necessitating the resection of surrounding organs. If resection of significant organs is needed, intra-abdominal marsupialization may be performed [
6]. The development of laparoscopic instruments and techniques in pediatric surgery has enabled the laparoscopic resection of abdominal lymphatic malformations [
22]. Of our 12 patients, 3 underwent laparoscopic resection.
Systemic drug therapy and OK-432 sclerotherapy may be considered as alternatives to surgical treatment for lymphatic malformation [
23242526]. Drugs can be administered safely and effectively without the complications or morbidities associated with surgery. Sclerotherapy, however, can be performed when lesions are located in superficial areas, such as the head and limbs, but there is insufficient data to develop a treatment algorithm for abdominal lymphatic malformations [
4]. It is difficult to administer drugs to the site of abdominal lymphatic malformations, and there is a risk of drug leakage into the abdominal cavity. Sclerotherapy may have minimal effects in the treatment of microcystic type lymphatic malformations and may be associated with complications, requiring careful judgment and thorough monitoring. Systemic treatment with drugs such as propranolol and sirolimus may be useful if extensive mesenteric involvement cannot be completely resected.
Patients undergoing complete surgical resection of abdominal lymphatic malformations have a good prognosis. Incomplete resection, however, may be associated with lesion recurrence and invasion of adjacent organs and structures. The recurrence rate of 9.5% after incomplete resection suggests that complete surgical resection is necessary to prevent recurrence [
14]. During follow-up, one (patient No. 6) of our 12 patients (8.3%) experienced lesion recurrence despite undergoing complete resection. Interestingly, 2 patients (patients No. 8 and 9) with extensive lesions who underwent incomplete resection showed complete lesion disappearance, with no evidence of recurrence.
In conclusion, although abdominal lymphatic malformations are benign, most children present with acute abdominal symptoms, necessitating early surgical treatment. Complete surgical excision was enough to cure most of the patients. After complete excision, there was no recurrence. Long-term follow-up of larger numbers of patients is needed to accurately evaluate the recurrence rate of abdominal lymphatic malformations.






 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download