1. Pierro A. Metabolism and nutritional support in the surgical neonate. J Pediatr Surg. 2002; 37:811–822.

2. Andorsky DJ, Lund DP, Lillehei CW, Jaksic T, Dicanzio J, Richardson DS, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001; 139:27–33.

3. Teitelbaum DH, Coran AG. Perioperative nutritional support in pediatrics. Nutrition. 1998; 14:130–142.

4. Koletzko B, Goulet O, Hunt J, Krohn K, Shamir R, et al. Parenteral Nutrition Guidelines Working Group. 1. Guidelines on paediatric parenteral nutrition of the European Society of Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and the European Society for Clinical Nutrition and Metabolism (ESPEN), supported by the European Society of Paediatric Research (ESPR). J Pediatr Gastroenterol Nutr. 2005; 41:Suppl 2. S1–S87.
5. Little DC, Rescorla FJ, Grosfeld JL, West KW, Scherer LR, Engum SA. Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. J Pediatr Surg. 2003; 38:852–856.

6. Nicholson GT, Clabby ML, Kanter KR, Mahle WT. Caloric intake during the perioperative period and growth failure in infants with congenital heart disease. Pediatr Cardiol. 2013; 34:316–321.

7. Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005; 115:696–703.

8. Mitting R, Marino L, Macrae D, Shastri N, Meyer R, Pathan N. Nutritional status and clinical outcome in postterm neonates undergoing surgery for congenital heart disease. Pediatr Crit Care Med. 2015; 16:448–452.

9. McLeod G, Sherriff J. Preventing postnatal growth failure--the significance of feeding when the preterm infant is clinically stable. Early Hum Dev. 2007; 83:659–665.
10. Owens JL, Musa N. Nutrition support after neonatal cardiac surgery. Nutr Clin Pract. 2009; 24:242–249.

11. Martin CR, Brown YF, Ehrenkranz RA, O'Shea TM, Allred EN, Belfort MB, et al. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics. 2009; 124:649–657.

12. Meurling S. The perioperative nutritional care of neonates and infants. Scand J Nutr. 2000; 44:8–11.

13. Cole CR, Hansen NI, Higgins RD, Ziegler TR, Stoll BJ. Eunice Kennedy Shriver NICHD Neonatal Research Network. Very low birth weight preterm infants with surgical short bowel syndrome: incidence, morbidity and mortality, and growth outcomes at 18 to 22 months. Pediatrics. 2008; 122:e573–e582.

14. Escribano J, Luque V, Ferre N, Mendez-Riera G, Koletzko B, Grote V, et al. Effect of protein intake and weight gain velocity on body fat mass at 6 months of age: the EU Childhood Obesity Programme. Int J Obes (Lond). 2012; 36:548–553.








 PDF
PDF ePub
ePub Citation
Citation Print
Print


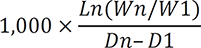


 XML Download
XML Download