Abstract
Purpose
Materials and Methods
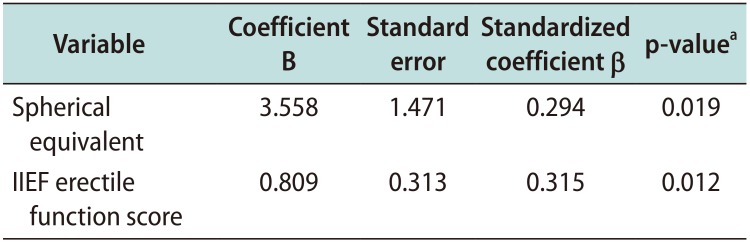
Results
ACKNOWLEDGEMENTS
Notes
Author Contribution:
Conceptualization: Kim YK, Yang DY.
Data curation: all authors.
Formal analysis: Kim YK.
Funding acquisition: Kim YK.
Investigation: all authors.
Methodology: all authors.
Supervision: Kim YK, Park SP, Yang DY.
Validation: all authors.
Writing (original draft): all authors.
Writing (review & editing): all authors.
References
Fig. 1
Choroidal thickness (CT) measurements. CT (yellow dashed arrows) was measured perpendicularly from the outer border of the retinal pigment epithelium to the inner sclera (yellow line) at three locations: at the fovea, 750 µm temporal to the fovea, and 750 µm nasal to the fovea. Blue asterisks represent the large choroidal vessel located close to the CT measurement locations. Large-choroidal-vessel-layer (LCVL) thickness (orange dashed arrows) was measured from the innermost point of the large choroidal vessel (green dashed lines) to the inner sclera. Small-choroidal-vessellayer (SCVL) thickness (red arrows) was calculated by subtracting LCVL thickness from total CT. We averaged the CT measured at three locations, i.e., subfoveal, temporal, and nasal locations, and used this average value for analysis.
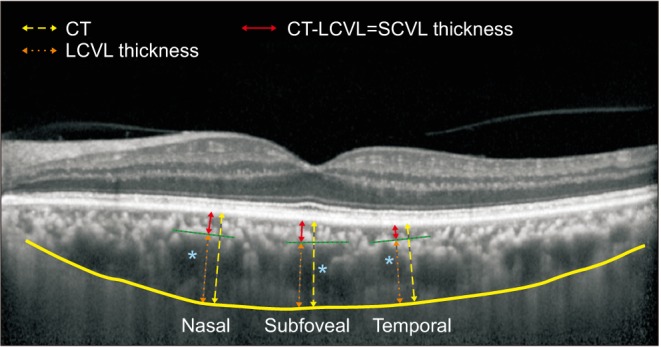
Table 1
Comparison of demographics and CT between patients in the control and erectile dysfunction groups
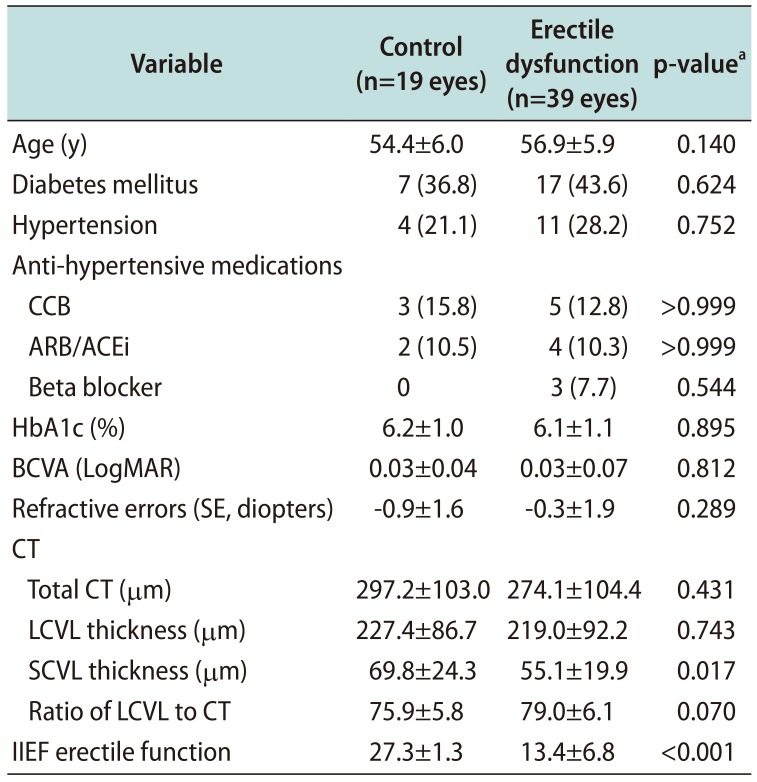
Values are presented as mean±standard deviation or number (%).
CT: choroidal thickness, CCB: calcium channel blocker, ARB/ACEi: angiotensin II receptor blocker/angiotensin converting enzyme inhibitor, HbA1c: hemoglobin A1c, BCVA: best-corrected visual acuity, LogMAR: logarithm of the minimum angle of resolution, SE: spherical equivalent, LCVL: large-choroidal-vessel-layer, SCVL: small-choroidal-vessel-layer, IIEF: International Index of Erectile Function.
aStudent t-test and chi-square test or Fisher's exact test were used for continuous and categorical variables, respectively.
Table 2
Comparison of demographics and CT between patients with and without diabetes mellitus
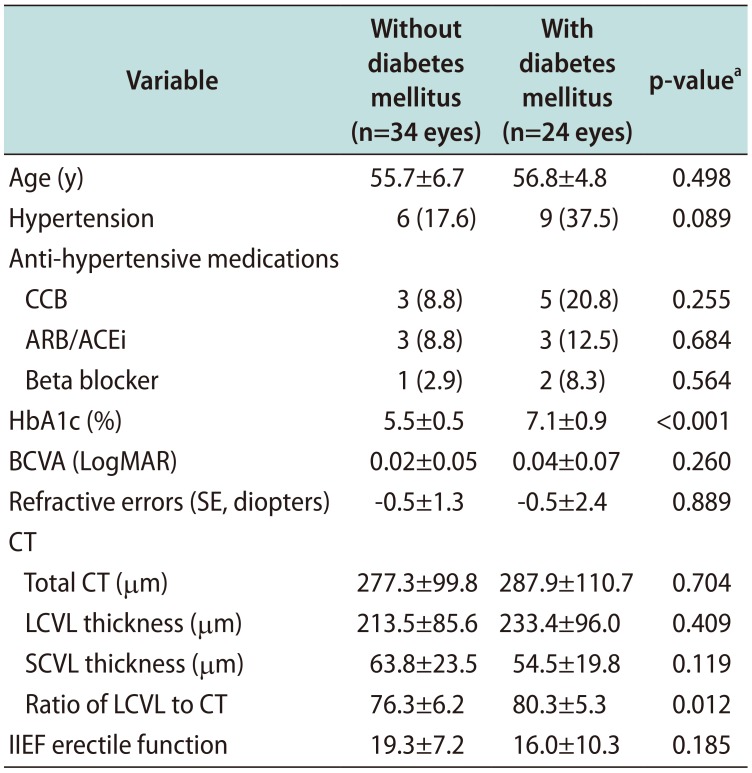
Values are presented as mean±standard deviation or number (%).
CT: choroidal thickness, CCB: calcium channel blocker, ARB/ACEi: angiotensin II receptor blocker/angiotensin converting enzyme inhibitor, HbA1c: hemoglobin A1c, BCVA: best-corrected visual acuity, LogMAR: logarithm of the minimum angle of resolution, SE: spherical equivalent, LCVL: large-choroidal-vessel-layer, SCVL: small-choroidal-vessel-layer, IIEF: International Index of Erectile Function.
aStudent t-test and chi-square test or Fisher's exact test were used for continuous and categorical variables, respectively.
Table 3
Comparison of demographics and CT between patients in the control and ED groups according to the presence or absence of DM
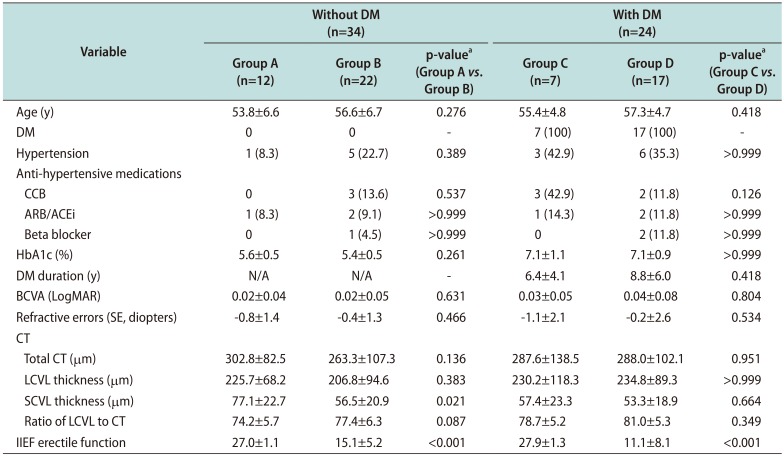
Values are presented as mean±standard deviation or number (%).
CT: choroidal thickness, ED: erectile dysfunction, DM: diabetes mellitus, Group A and Group C: No ED, Group B and Group D: ED, CCB: calcium channel blocker, ARB/ACEi: angiotensin II receptor blocker/angiotensin converting enzyme inhibitor, HbA1c: hemoglobin A1c, BCVA: best-corrected visual acuity, LogMAR: logarithm of the minimum angle of resolution, SE: spherical equivalent, LCVL: large-choroidal-vessel-layer, SCVL: small-choroidal-vessel-layer, IIEF: International Index of Erectile Function, N/A: not available.
aMann-Whitney U-test and chi-square test or Fisher's exact test were used for continuous and categorical variables, respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download