SMOFlipid (Fresenius Kabi, Bad Homburg, Germany) is a mixture of different lipid emulsions consisting of soybean oil, medium-chain triglyceride, olive oil, and fish oil. It is indicated as a source of calories and essential fatty acids for parenteral nutrition when oral or enteral nutrition is not possible, insufficient, or contraindicated.
1
At a neonatal intensive care unit of a university hospital in Seoul, four neonates died consecutively within a time span of 80 minutes on 16 December 2017.
Citrobacter freundii was isolated from the blood samples from the 4 neonates. In 3 of the 4 neonates, the bacterium was cultured from the cerebrospinal fluids obtained during an autopsy study. It was also isolated from the SMOFlipid that had been infused to the neonates. The sequences of the antibiotic resistance gene of
C. freundii were reported to be identical between the clinical isolates and the lipid emulsion isolate.
2
Possible explanations for the sudden deaths of the 4 neonates within such a short time span may include 1) fulminant sepsis due to direct infusion of a high inoculum of C. freundii, 2) pulmonary embolism due to fat globules in the lipid emulsion. In this in vitro study, we evaluated the bacterial growth kinetics and increase in number and size of fat globules in SMOFlipid contaminated with C. freundii.
For the bacterial growth kinetics, the bacterial strains of C. freundii ATCC 8090, Escherichia coli ATCC 25922, and methicillin-resistant Staphylococcus aureus (MRSA) N315 were used. For the lipid formulation, SMOFlipid 20% infusion 100 mL (Fresenius Kabi) was used. In order to compare growth kinetics of C. freundii in different intravenous fluids for nutritional supplements, normal saline (CJ Healthcare, Seoul, Korea), amino acids (Primene 10%, Baxter Healthcare, Deerfield, IL, USA), and glucose (5% dextrose; CJ Healthcare) were also used.
The bacterial strains were cultured overnight at 37°C in blood agar plate (BAP). One of each bacterial colony was diluted in 10 mL of Brain Heart Infusion broth, 10 µL of which was inoculated in 10 mL of SMOFlipid. Then, the inoculated SMOFlipid was cultured at room temperature. At 0, 1, 2, 4, 6, and 24 hours after bacterial inoculation, SMOFlipid was serially diluted and plated onto BAP and incubated at 37°C for 24 hours. Colonies were counted and data were expressed as viable colony forming unit (CFU)/mL. SMOFlipid without bacterial inoculation was also tested as a control. All experiments were performed in triplicate. To measure the pH values of the SMOFlipids, we used Accumet XL15 pH Meter (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
To assess the change in number and size of fat globules, 10 µL of gently agitated sample was transferred to a microscope slide, and covered with a cover slide. All of the sample field was inspected with final magnification of × 400 using Leica DM IL LED phase-contrast microscope, and typical microscopic depictions were captured with Leica DFC295 camera and Leica Application Suite Version 3.8.0 (Leica Microsystems, Heerbrugg, Switzerland). We also observed fat globules after filtering the SMOFlipids with PALL Lipopor NLF2E filter (Pall Medical, Port Washington, NY, USA), a 1.2 micrometer in-line filter.
To elucidate the effect of admixture of antibiotics with SMOFlipid on fat globules, vancomycin (CJ Healthcare), gentamicin (Shin Poong Pharmaceuticals, Seoul, Korea), ampicillin (Yung Jin Pharmaceutical, Seoul, Korea), and cefotaxime (CJ Healthcare) were used. The antibiotics were serially diluted from maximal dilution concentrations to usual therapeutic levels in blood.
3 The changes in number and size of fat globules were observed 10 minutes after the admixture using Leica DM IL LED phase-contrast microscope. The experiment was repeated twice.
The growth kinetics of
C. freundii,
E. coli, and MRSA in SMOFlipid are shown in
Fig. 1A. The number of
C. freundii and
E. coli increased very rapidly, and reached > 10
6 CFU/mL at 24 hours after the inoculation. However, MRSA did not grow well in SMOFlipid. The pH of SMOFlipid decreased over time as the number of
C. freundii increased, and it was < 6.0 at time 24 hours. In contrast, it rarely changed in SMOFlipid inoculated with MRSA (
Fig. 1B). The growth kinetics of
C. freundii in different fluids are shown in
Fig. 1C. It grew rapidly in SMOFlipid but not as much in 10% amino acid, 5% glucose, and normal saline.
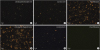
The number of fat globules with > 5 µm increased over time in SMOFlipid inoculated with
C. freundii (
Fig. 2). By 24 hours after the inoculation, many of the large fat globules in SMOFlipid inoculated with
C. freundii were > 20 µm in diameter and the size of fat globules increased up to 40 µm. After the filtration of SMOFlipid with the 1.2 micrometer in-line filter, all the large fat globules were not observed.
The number of fat globules with > 5 µm in diameter also increased when SMOFlipid was mixed with vancomycin and gentamicin. However, it did not in SMOFlipid mixed with ampicillin and cefotaxime (
Supplementary Fig. 1). Precipitations were also observed when gentamicin with a concentration of 1 mg/mL was mixed with SMOFlipid.
Our study demonstrated that C. freundii grew rapidly in SMOFlipid, and its number increased up to > 106 CFU/mL by 24 hours after contamination. The bacterium grew more rapidly in the lipid emulsion than in 5% glucose or 10% amino acid solution. We also showed that fat globules in SMOFlipid increased in size and number over time after the bacterial contamination.
Bacterial contamination is an important safety issue involving lipid emulsions, because the iso-osmotic and nutritional properties of lipid emulsions are ideal environment for bacterial growth.
4 Previous studies also reported the association of intravenous lipid emulsion and outbreaks of bacteremia at neonatal intensive care unit.
567 The bacterial contaminations occurred while manipulating the “stock bottle” to withdraw aliquots for different patients.
56 The smallest unit volume of SMOFlipid available is 100 mL per bottle, and the usual dose for neonates is < 20 mL per day; therefore, the bottle may be entered on multiple occasions to withdraw 20 mL aliquots. Once bacterial contamination occur during manipulation, some bacteria such as
Citrobacter may proliferate rapidly in SMOFlipid, as shown in our study.
Infusion of a high inoculum of bacteria into blood stream may result in a fulminant sepsis, especially in preterm infants whose immune systems are severely impaired. Indeed, the autopsy studies of the 4 neonates showed isolation of
C. freundii from the blood samples in 4/4 neonates, and from the cerebrospinal fluids in 3/4 neonates,
2 suggesting fulminant sepsis may be the direct causes of the deaths.
Another safety issue involving lipid emulsions is instability of fat droplets in the emulsions. The median droplet size of lipid injectable emulsions is < 1.0 µm.
8 These emulsions are thermodynamically unstable, and the lipid droplets may aggregate to grow into large unstable globules with > 5 µm in diameter. These fat globules may cause fat embolism by obstructing small capillaries in the lungs (internal diameter 4–9 µm).
489 The physicochemical stability of lipid emulsions may be compromised, especially when they are infused during acute metabolic stress, as in the critical care setting.
10 In particular, preterm infants and neonates who are critically ill have poor plasma clearance and increased lipid levels, and possibly lead to pulmonary fat embolism,
11 or pulmonary fat accumulation.
12 Because of this safety issue due to the instability of lipid emulsions, especially in preterm infants, the US manufacturer's product package insert prominently notes the black box warning of death in preterm infants.
8
The prime destabilizers of lipid emulsions are excessive acidity and inappropriate electrolyte contents.
4 Our data suggest that bacterial contamination may also destabilize lipid emulsions, forming large fat globules via coalescence of lipid droplets. Possible mechanism of this change may be the decrease in pH due to the metabolites of bacterial growth, and as the pH decreases, excessive acidity may neutralize zeta potential (a net negative charge of lipid droplets) that exert electrostatic repulsive force to prevent coalescence.
4 In our study, the bacterial contamination decreased the pH of SMOFlipid to < 6.0, the lower limit of pH for lipid injectable emulsions specified by US pharmacopeia.
8 It is of note that the intravenous solutions of 5% glucose, 10% amino acid, and normal saline are acidic: the pH values for them are 4.3, 5.5, and 5.6, respectively.
131415 Therefore, mixing these solutions with lipid emulsions may also destabilize lipid emulsions.
Limitations of our study include measurement of particle size distribution by microscopy, instead of a more sophisticated particle sizing technique, such as laser light extinction or nephelometry. Although microscopy may not detect changes in distribution of small lipid particles, it can detect the change when percentage of lipid globules > 5 µm exceed 4%, which is the worst case scenario for patient safety.
410 Another limitation is we did not perform an in vivo experiment to demonstrate fat embolism by
C. freundii-contaminated SMOFlipid. Because the autopsy study by the National Forensic Service did not find fat accumulation in the lung tissues of the neonates, fat embolism was not officially considered as direct cause of deaths. However, in order to rule out a possibility of fat embolism more clearly, in vivo experiment is required.
Based on our study findings, we propose that pulmonary fat embolism as well as fulminant sepsis may be a possible cause of the deaths of the 4 neonates. In order to prevent morbidity or mortality due to intravenous lipid emulsions, emulsion stability and sterility must be perfectly maintained. Manipulation of intravenous lipid emulsions should always be performed under strict aseptic pharmaceutical conditions, such as within a laminar airflow hood by well-trained pharmacy staff.
4 Admixture of other medications with and administration of lipid emulsions should follow the manufacturer's instructions, and a 1.2 micron in-line filter should be used during administration.
1





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download