1. Falkinham JO 3rd. Environmental sources of nontuberculous mycobacteria. Clin Chest Med. 2015; 36:35–41. PMID:
25676517.

2. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416. PMID:
17277290.

3. Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017; 72:ii1–ii64.

4. Daley CL. Mycobacterium avium complex disease. Microbiol Spectr. 2017; 5:TNMI7-0045-2017.
5. Koh WJ. Nontuberculous mycobacteria: overview. Microbiol Spectr. 2017; 5:TNMI7-0024-2016.
6. Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of nontuberculous mycobacterial lung disease: clinicians' perspectives. Tuberc Respir Dis. 2016; 79:74–84.

7. Kwon YS, Koh WJ. Diagnosis and treatment of nontuberculous mycobacterial lung disease. J Korean Med Sci. 2016; 31:649–659. PMID:
27134484.

8. Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013; 42:1604–1613. PMID:
23598956.

9. Moon SM, Kim SY, Jhun BW, Lee H, Park HY, Jeon K, et al. Clinical characteristics and treatment outcomes of pulmonary disease caused by
Mycobacterium chimaera. Diagn Microbiol Infect Dis. 2016; 86:382–384. PMID:
27720208.
10. Kim SY, Shin SH, Moon SM, Yang B, Kim H, Kwon OJ, et al. Distribution and clinical significance of
Mycobacterium avium complex species isolated from respiratory specimens. Diagn Microbiol Infect Dis. 2017; 88:125–137. PMID:
28291631.
11. Schweickert B, Goldenberg O, Richter E, Gobel UB, Petrich A, Buchholz P, et al. Occurrence and clinical relevance of
Mycobacterium chimaera sp. nov., Germany. Emerg Infect Dis. 2008; 14:1443–1446. PMID:
18760016.
12. Boyle DP, Zembower TR, Reddy S, Qi C. Comparison of clinical features, virulence, and relapse among
Mycobacterium avium complex species. Am J Respir Crit Care Med. 2015; 191:1310–1317. PMID:
25835090.
13. Kitada S, Uenami T, Yoshimura K, Tateishi Y, Miki K, Miki M, et al. Long-term radiographic outcome of nodular bronchiectatic
Mycobacterium avium complex pulmonary disease. Int J Tuberc Lung Dis. 2012; 16:660–664. PMID:
22410245.
14. Hayashi M, Takayanagi N, Kanauchi T, Miyahara Y, Yanagisawa T, Sugita Y. Prognostic factors of 634 HIV-negative patients with
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012; 185:575–583. PMID:
22199005.
15. Lee G, Lee KS, Moon JW, Koh WJ, Jeong BH, Jeong YJ, et al. Nodular bronchiectatic
Mycobacterium avium complex pulmonary disease: natural course on serial computed tomographic scans. Ann Am Thorac Soc. 2013; 10:299–306. PMID:
23952847.
16. Gochi M, Takayanagi N, Kanauchi T, Ishiguro T, Yanagisawa T, Sugita Y. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open. 2015; 5:e008058.
17. Koh WJ, Moon SM, Kim SY, Woo MA, Kim S, Jhun BW, et al. Outcomes of
Mycobacterium avium complex lung disease based on clinical phenotype. Eur Respir J. 2017; 50:1602503. PMID:
28954780.
18. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013; 34:87–94. PMID:
23460008.

19. Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. 2015; 36:13–34. PMID:
25676516.
20. Ko RE, Moon SM, Ahn S, Jhun BW, Jeon K, Kwon OJ, et al. Changing epidemiology of nontuberculous mycobacterial lung diseases in a tertiary referral hospital in Korea between 2001 and 2015. J Korean Med Sci. 2018; 33:e65. PMID:
29441757.

21. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006; 129:341–348. PMID:
16478850.

22. Park YS, Lee CH, Lee SM, Yang SC, Yoo CG, Kim YW, et al. Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis. 2010; 14:1069–1071. PMID:
20626955.
23. Lee SK, Lee EJ, Kim SK, Chang J, Jeong SH, Kang YA. Changing epidemiology of nontuberculous mycobacterial lung disease in South Korea. Scand J Infect Dis. 2012; 44:733–738. PMID:
22720876.

24. Yoo JW, Jo KW, Kim MN, Lee SD, Kim WS, Kim DS, et al. Increasing trend of isolation of non-tuberculous mycobacteria in a tertiary university hospital in South Korea. Tuberc Respir Dis. 2012; 72:409–415.

25. Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between
Mycobacterium avium and
Mycobacterium intracellulare in
M avium complex lung disease. Chest. 2012; 142:1482–1488. PMID:
22628488.
26. Hwang JA, Kim S, Jo KW, Shim TS. Natural history of
Mycobacterium avium complex lung disease in untreated patients with stable course. Eur Respir J. 2017; 49:1600537. PMID:
28275170.
27. Pan SW, Shu CC, Feng JY, Wang JY, Chan YJ, Yu CJ, et al. Microbiological persistence in patients with
Mycobacterium avium complex lung disease: the predictors and the Impact on radiographic progression. Clin Infect Dis. 2017; 65:927–934. PMID:
28541556.
28. Henkle E, Novosad SA, Shafer S, Hedberg K, Siegel SAR, Ku J, et al. Long-term outcomes in a population-based cohort with respiratory nontuberculous mycobacteria isolation. Ann Am Thorac Soc. 2017; 14:1120–1128. PMID:
28406709.

29. Kim SJ, Park J, Lee H, Lee YJ, Park JS, Cho YJ, et al. Risk factors for deterioration of nodular bronchiectatic
Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2014; 18:730–736. PMID:
24903946.
30. Kim SJ, Yoon SH, Choi SM, Lee J, Lee CH, Han SK, et al. Characteristics associated with progression in patients with of nontuberculous mycobacterial lung disease : a prospective cohort study. BMC Pulm Med. 2017; 17:5. PMID:
28056937.

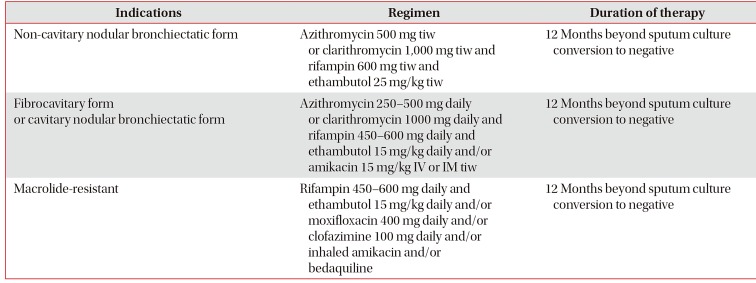
31. Wallace RJ Jr, Brown-Elliott BA, McNulty S, Philley JV, Killingley J, Wilson RW, et al. Macrolide/Azalide therapy for nodular/bronchiectatic
Mycobacterium avium complex lung disease. Chest. 2014; 146:276–282. PMID:
24457542.
32. Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015; 191:96–103. PMID:
25393520.
33. Adjemian J, Prevots DR, Gallagher J, Heap K, Gupta R, Griffith D. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc. 2014; 11:9–16. PMID:
24236749.

34. van Ingen J, Wagner D, Gallagher J, Morimoto K, Lange C, Haworth CS, et al. Poor adherence to management guidelines in nontuberculous mycobacterial pulmonary diseases. Eur Respir J. 2017; 49:1601855. PMID:
28182571.

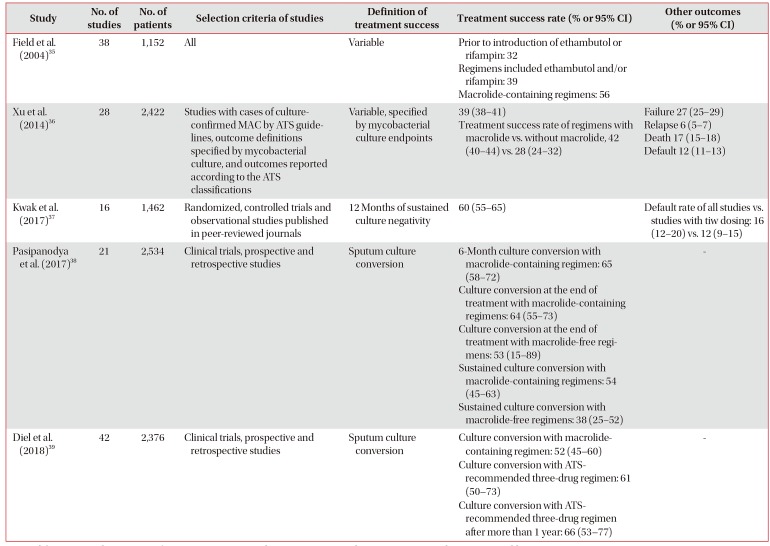
35. Field SK, Fisher D, Cowie RL.
Mycobacterium avium complex pulmonary disease in patients without HIV infection. Chest. 2004; 126:566–581. PMID:
15302746.
36. Xu HB, Jiang RH, Li L. Treatment outcomes for
Mycobacterium avium complex: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2014; 33:347–358. PMID:
23979729.
37. Kwak N, Park J, Kim E, Lee CH, Han SK, Yim JJ. Treatment outcomes of
Mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis. 2017; 65:1077–1084. PMID:
28582488.
38. Pasipanodya JG, Ogbonna D, Deshpande D, Srivastava S, Gumbo T. Meta-analyses and the evidence base for microbialoutcomes in the treatment of pulmonary
Mycobacterium avium-intracellulare complex disease. J Antimicrob Chemother. 2017; 72(Suppl_2):i3–i19. PMID:
28922813.
39. Diel R, Nienhaus A, Ringshausen FC, Richter E, Welte T, Rabe KF, et al. Microbiologic outcome of interventions against
Mycobacterium avium complex pulmonary disease: a systematic review. Chest. 2018; 153:888–921. PMID:
29410162.
40. Griffith DE, Adjemian J, Brown-Elliott BA, Philley JV, Prevots DR, Gaston C, et al. Semiquantitative culture analysis during therapy for
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015; 192:754–760. PMID:
26068042.
41. Loebinger MR.
Mycobacterium avium complex infection: phenotypes and outcomes. Eur Respir J. 2017; 50:1701380. PMID:
28954776.
42. Griffith DE, Brown-Elliott BA, Shepherd S, McLarty J, Griffith L, Wallace RJ Jr. Ethambutol ocular toxicity in treatment regimens for
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2005; 172:250–253. PMID:
15860751.
43. Lam PK, Griffith DE, Aksamit TR, Ruoss SJ, Garay SM, Daley CL, et al. Factors related to response to intermittent treatment of
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006; 173:1283–1289. PMID:
16514112.
44. Jeong BH, Jeon K, Park HY, Moon SM, Kim SY, Lee SY, et al. Peak plasma concentration of azithromycin and treatment responses in
Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2016; 60:6076–6083. PMID:
27480854.
45. Philley JV, Griffith DE. Treatment of slowly growing mycobacteria. Clin Chest Med. 2015; 36:79–90. PMID:
25676521.

46. Jhun BW, Kim SY, Moon SM, Jeon K, Kwon OJ, Huh HJ, et al. Development of macrolide resistance and reinfection in refractory
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2018; 198:1322–1330. PMID:
29877739.
47. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT, Onyi GO, et al. Initial clarithromycin monotherapy for
Mycobacterium avium-intracellulare complex lung disease. Am J Respir Crit Care Med. 1994; 149:1335–1341. PMID:
8173775.
48. Griffith DE, Brown BA, Girard WM, Murphy DT, Wallace RJ Jr. Azithromycin activity against
Mycobacterium avium complex lung disease in patients who were not infected with human immunodeficiency virus. Clin Infect Dis. 1996; 23:983–989. PMID:
8922790.
49. Wallace RJ Jr, Brown BA, Griffith DE, Girard WM, Murphy DT. Clarithromycin regimens for pulmonary
Mycobacterium avium complex. The first 50 patients. Am J Respir Crit Care Med. 1996; 153(6 Pt 1):1766–1772. PMID:
8665032.
50. Griffith DE, Brown BA, Murphy DT, Girard WM, Couch L, Wallace RJ Jr. Initial (6-month) results of three-times-weekly azithromycin in treatment regimens for
Mycobacterium avium complex lung disease in human immunodeficiency virus-negative patients. J Infect Dis. 1998; 178:121–126. PMID:
9652431.
51. Jhun BW, Moon SM, Kim SY, Park HY, Jeon K, Kwon OJ, et al. Intermittent antibiotic therapy for recurrent nodular bronchiectatic
Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2018; e01812-17. PMID:
29203483.
52. Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, et al. Clinical and molecular analysis of macrolide resistance in
Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006; 174:928–934. PMID:
16858014.
53. Kadota T, Matsui H, Hirose T, Suzuki J, Saito M, Akaba T, et al. Analysis of drug treatment outcome in clarithromycinresistant
Mycobacterium avium complex lung disease. BMC Infect Dis. 2016; 16:31. PMID:
26818764.

54. Moon SM, Park HY, Kim SY, Jhun BW, Lee H, Jeon K, et al. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant
Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2016; 60:6758–6765. PMID:
27572413.
55. Morimoto K, Namkoong H, Hasegawa N, Nakagawa T, Morino E, Shiraishi Y, et al. Macrolide-resistant
Mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc. 2016; 13:1904–1911. PMID:
27513168.
56. Koh WJ, Hong G, Kim SY, Jeong BH, Park HY, Jeon K, et al. Treatment of refractory
Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother. 2013; 57:2281–2285. PMID:
23478956.
57. Kang HK, Park HY, Kim D, Jeong BH, Jeon K, Cho JH, et al. Treatment outcomes of adjuvant resectional surgery for nontuberculous mycobacterial lung disease. BMC Infect Dis. 2015; 15:76. PMID:
25887191.

58. Asakura T, Hayakawa N, Hasegawa N, Namkoong H, Takeuchi K, Suzuki S, et al. Long-term outcome of pulmonary resection for nontuberculous mycobacterial pulmonary disease. Clin Infect Dis. 2017; 65:244–251. PMID:
28369361.

59. Mitchell JD, Bishop A, Cafaro A, Weyant MJ, Pomerantz M. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg. 2008; 85:1887–1892. PMID:
18498789.

60. Mitchell JD. Surgical approach to pulmonary nontuberculous mycobacterial infections. Clin Chest Med. 2015; 36:117–122. PMID:
25676524.

61. Tang S, Yao L, Hao X, Liu Y, Zeng L, Liu G, et al. Clofazimine for the treatment of multidrug-resistant tuberculosis: prospective, multicenter, randomized controlled study in China. Clin Infect Dis. 2015; 60:1361–1367. PMID:
25605283.

62. Field SK, Cowie RL. Treatment of
Mycobacterium avium-intracellulare complex lung disease with a macrolide, ethambutol, and clofazimine. Chest. 2003; 124:1482–1486. PMID:
14555583.
63. Jarand J, Davis JP, Cowie RL, Field SK, Fisher DA. Long-term follow-up of
Mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or rifampin. Chest. 2016; 149:1285–1293. PMID:
26513209.
64. Martiniano SL, Wagner BD, Levin A, Nick JA, Sagel SD, Daley CL. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest. 2017; 152:800–809. PMID:
28483608.

65. Olivier KN, Shaw PA, Glaser TS, Bhattacharyya D, Fleshner M, Brewer CC, et al. Inhaled amikacin for treatment of refractory pulmonary nontuberculous mycobacterial disease. Ann Am Thorac Soc. 2014; 11:30–35. PMID:
24460437.

66. Yagi K, Ishii M, Namkoong H, Asami T, Iketani O, Asakura T, et al. The efficacy, safety, and feasibility of inhaled amikacin for the treatment of difficult-to-treat non-tuberculous mycobacterial lung diseases. BMC Infect Dis. 2017; 17:558. PMID:
28793869.

67. Jhun BW, Yang B, Moon SM, Lee H, Park HY, Jeon K, et al. Amikacin inhalation as salvage therapy for refractory nontuberculous mycobacterial lung disease. Antimicrob Agents Chemother. 2018; e00011-18. PMID:
29661870.

68. Rose SJ, Neville ME, Gupta R, Bermudez LE. Delivery of aerosolized liposomal amikacin as a novel approach for the treatment of nontuberculous mycobacteria in an experimental model of pulmonary infection. PLoS One. 2014; 9:e108703. PMID:
25264757.

69. Olivier KN, Griffith DE, Eagle G, McGinnis JP 2nd, Micioni L, Liu K, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2017; 195:814–823. PMID:
27748623.

70. Griffith DE, Eagle G, Thomson R, Aksamit TR, Hasegawa N, Morimoto K, et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by
Mycobacterium avium complex (CONVERT): a prospective, open-label, randomized study. Am J Respir Crit Care Med. 2018; 9. 14. [Epub]. DOI:
10.1164/rccm.201807-1318OC.
71. Kwon YS, Jeong BH, Koh WJ. Tuberculosis: clinical trials and new drug regimens. Curr Opin Pulm Med. 2014; 20:280–286. PMID:
24614239.
72. Kwon YS, Koh WJ. Synthetic investigational new drugs for the treatment of tuberculosis. Expert Opin Investig Drugs. 2016; 25:183–193.

73. Brown-Elliott BA, Philley JV, Griffith DE, Thakkar F, Wallace RJ Jr.
In vitro susceptibility testing of bedaquiline against
Mycobacterium avium complex. Antimicrob Agents Chemother. 2017; 61:e01798-16. PMID:
27872065.

74. Vesenbeckh S, Schonfeld N, Krieger D, Bettermann G, Bauer TT, Russmann H, et al. Bedaquiline as a potential agent in the treatment of
M. intracellulare and
M. avium infections. Eur Respir J. 2017; 49:1601969. PMID:
28331039.
75. Philley JV, Wallace RJ Jr, Benwill JL, Taskar V, Brown-Elliott BA, Thakkar F, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest. 2015; 148:499–506. PMID:
25675393.

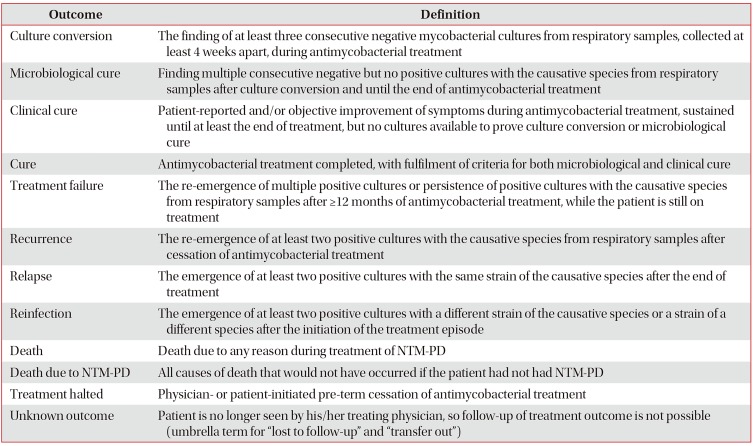
76. van Ingen J, Aksamit T, Andrejak C, Bottger EC, Cambau E, Daley CL, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur Respir J. 2018; 51:1800170. PMID:
29567726.

77. Satta G, McHugh TD, Mountford J, Abubakar I, Lipman M. Managing pulmonary nontuberculous mycobacterial infection. time for a patient-centered approach. Ann Am Thorac Soc. 2014; 11:117–121. PMID:
24460445.

78. Mehta M, Marras TK. Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med. 2011; 105:1718–1725. PMID:
21868209.

79. Lee MR, Yang CY, Chang KP, Keng LT, Yen DH, Wang JY, et al. Factors associated with lung function decline in patients with non-tuberculous mycobacterial pulmonary disease. PLoS One. 2013; 8:e58214. PMID:
23483998.

80. Park HY, Jeong BH, Chon HR, Jeon K, Daley CL, Koh WJ. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest. 2016; 150:1222–1232. PMID:
27298072.

81. Asakura T, Funatsu Y, Ishii M, Namkoong H, Yagi K, Suzuki S, et al. Health-related quality of life is inversely correlated with C-reactive protein and age in
Mycobacterium avium complex lung disease: a cross-sectional analysis of 235 patients. Respir Res. 2015; 16:145. PMID:
26635226.

82. Hama M, Ushiki A, Kosaka M, Yamazaki Y, Yasuo M, Yamamoto H, et al. Health-related quality of life in patients with pulmonary non-tuberculous mycobacteria infection. Int J Tuberc Lung Dis. 2016; 20:747–752. PMID:
27155176.

83. Asakura T, Ishii M, Ishii K, Suzuki S, Namkoong H, Okamori S, et al. Health-related QOL of elderly patients with pulmonary
M. avium complex disease in a university hospital. Int J Tuberc Lung Dis. 2018; 22:695–703. PMID:
29862956.
84. Czaja CA, Levin AR, Cox CW, Vargas D, Daley CL, Cott GR. Improvement in quality of life after therapy for
Mycobacterium abscessus group lung infection: a prospective cohort study. Ann Am Thorac Soc. 2016; 13:40–48. PMID:
26540302.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download