Abstract
Background
Iron overload is a risk factor affecting all patients with thalassemia intermedia (TI). We aimed to determine whether there is a relationship of serum ferritin (SF) and alanine aminotransferase (ALT) with liver iron concentration (LIC) determined by R2 magnetic resonance imaging (R2-MRI), to estimate the most relevant degree of iron overload and best time to chelate in patients with TI.
Methods
In this cross-sectional study, 119 patients with TI (mean age years) were randomly selected and compared with 120 patients who had a diagnosis of thalassemia major (TM). Correlations of LIC, as determined by R2-MRI, with SF and ALT levels, were assessed in all participants. A P-value <0.05 was considered statistically significant.
Results
SF and LIC levels were lower in patients with TI than in those with TM; only ferritin values were significant. We found a statistically significant relationship between SF and LIC, with cut-off estimates of SF in patients with TI who had splenectomy and those who entered puberty spontaneously (916 and 940 ng/mL, respectively) with LIC >5 mg Fe/g dry weight (P<0.0001). A significant relationship was also found for patients with TI who had elevated ALT level (63.5 U/L), of 3.15 times the upper normal laboratory limit, using a cut-off for LIC ≥5 mg Fe/g dry weight.
Go to : 
The field of thalassemia is wide ranging, from thalassemia minor, which is characterized by mild hypochromic microcytic anemia without apparent clinical features, to thalassemia major (TM), which is recognized by severe anemia that starts in the first years of life and is transfusion dependent. Thalassemia intermedia (TI) describes patients with mild or moderate anemia [1]. Patients with TI usually do not need regular or frequent blood transfusion; thus, TI is a type of non-transfusion dependent thalassemia (NTDT) [23]. However, TI is not free of complications related to iron accumulation, in which hemostasis and hepcidin regulation are affected [45]. Iron is absorbed at higher rates through the intestinal mucosa in TI, promoting iron loading in different body tissues; if not treated with chelating therapies, iron toxicity develops, leading to the failure of various vital organs and finally death [56]. To measure iron overload, serum ferritin (SF) is commonly used as it is usually elevated during iron accumulation. Use of SF is a simple, easily available method that can be performed repeatedly and at a low cost [78]. The liver iron concentration (LIC) declines after chelating therapies, which is associated with fewer complications [79]. LIC has been traditionally measured via tissue biopsy. Recently, a noninvasive method has been developed using R2-magnetic resonance imaging (MRI). Good correlation has been found between LIC and SF in transfusion-dependent thalassemia; however, the data on NTDT are limited and include some conflicting results. Nevertheless, SF is not a good predictor of LIC in TI or other NTDTs [121011].
The purpose of our study was to investigate the relationship between LIC determined by R2-MRI, SF levels, and liver enzymes represented by alanine aminotransferase (ALT), in patients with TI. We also aimed to develop an inexpensive and easy method for detecting iron overload requiring chelation in a special group of patients with TI (related to either the spleen, puberty status, and/or liver enzyme values). The data reported here represent the largest investigation of this correlation in TI; our findings will thus provide valuable information on the relationship among these parameters in this specific patient population.
Go to : 
This was a cross-sectional study of randomly selected patients with TI managed at Hereditary Anemia Center at our teaching hospital from the 1 August 2015 until the end of June in 2017.
We performed a complete review of medical records, including information on age; blood transfusion history; hemoglobin concentration particularly pre-transfusion levels; and surgical history, especially splenectomy status. Data from a randomly selected population of patients with TM treated at our center were also obtained for comparative evaluation. The patients in both groups were well matched for age and gender.
At the start of the study, blood samples were obtained to assess non-fasting SF levels using an electrofluorescence assay method with a MINI VIDAS analyzer (bioMerieux S.A., Lyon, France). In patients with thalassemia, LIC is 1 of the best indicators of iron overload, which is related to many organic complications. To prevent any delay in the management of iron overload in patients with TI, we used a value of >5 mg Fe/g dry weight LIC as a cut-off estimate. Direct determination of LIC was done using R2-MRI. MRI measurements were performed using a 4-element body coil on a 3 Tesla MRI scanner (MAGNETOM Spectra; Siemens Healthineers, Malvern, PA, USA) and Medis Suite software (Medis Medical Imaging Systems B.V., Leiden, the Netherlands). MRI results were assessed by 1 expert radiologist.
Stages of puberty were assessed in all patients according to the Tanner scale [12]. A specialized gynecologist and endocrinologist took part in patient examinations. Failed spontaneous onset of puberty was defined as the absence of breast development in girls at 14 years of age and of testicular enlargement in boys at age 16 years or more [13]. Arrested puberty was defined as a lack of puberty progress for >1 year, without progress to Tanner stage 4/5 beyond 16 years of age. Secondary amenorrhea was identified when girls missed menstrual cycles for a year or longer after menarche [1415].
Patients with any infections, fever, or a positive history of viral hepatitis (hepatitis B or C virus), found while checking SF levels, as well as overt heart and liver failure, were excluded from the study. Among our selected patients, liver enzymes (ALT) were less than 10 times the upper normal laboratory limit, and platelet counts were within usual laboratory findings. According to the above criteria, we recruited 119 patients with a diagnosis of TI ≥2 years of age. Another 120 patients with TM were also included for comparison purposes, using random selection based on the same criteria as above.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the hospital's ethics and scientific research committee (registration code: 24/2015). Informed consent was obtained from all patients involved in the study, or their caregivers, after being informed that their personal health information would be safeguarded.
Descriptive statistics were first used to record data and were presented as mean ± standard deviation (SD). The chi-square test was then applied to determine the associations between related categorical variables, and the Student t-test was used to detect differences between means of numerical variables. The Pearson correlation test (R) was used to assess relationships among the studied parameters. Receiver operating characteristic (ROC) curve analysis was performed to determine the optimum level of a specific parameter, to predict abnormal values of the corresponding parameter. All statistical analyses were conducted using IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA). A P-value <0.05 was considered to be significant.
Go to : 
In this study, we included 119 patients with TI and a similar number of patients with TM (N=120). Patients with TI had mean age comparable to those with TM. Pre-transfusion hemoglobin levels were higher in patients with TI than in those with TM but did not reach a statistically significant level. Slightly more than a quarter of patients with TI (28.57%) had undergone splenectomy, in comparison with 43.33% in patients who had TM. Delayed puberty (or any other abnormality such as arrested puberty or secondary amenorrhea) was found in 82 (68.91%) patients with TI whereas 37 (31.09%) patients had spontaneous onset of puberty. For the TM group, 98 (81.67%) patients had delayed puberty or another abnormality related to the usual timing of pubertal progress whereas 22 (18.33%) patients had spontaneous puberty.
There was a statistically significant difference between mean SF levels in patients with TI and those with TM, with lower values for the TI group (1,096 vs. 2,319 ng/mL, P<0.0001). Mean LIC levels were lower in the TI group but without significance (9.6 vs. 10.1 mg Fe/g dry weight, P=0.5844). All the above general characteristics are presented in Table 1.
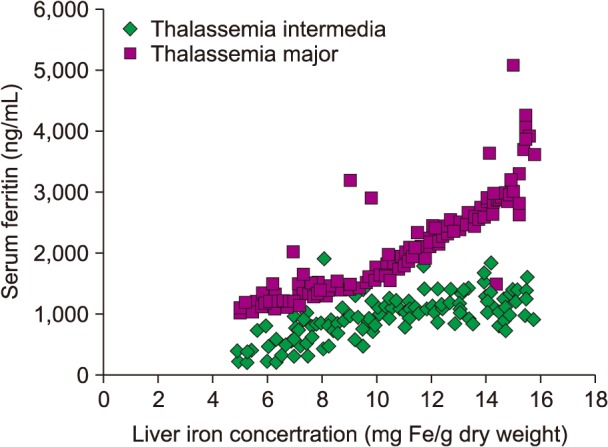
Fig. 1 shows the relationship between SF and LIC in both patient groups, TI and TM. We found a positive correlation between these 2 groups, with R=0.27 for TI and R=0.76 for TM; however, the TI group showed no statistically significant values, unlike the TM group (P=0.0631 vs. P<0.0001). SF levels were higher in patients with TM than in those with TI for similar LIC values.
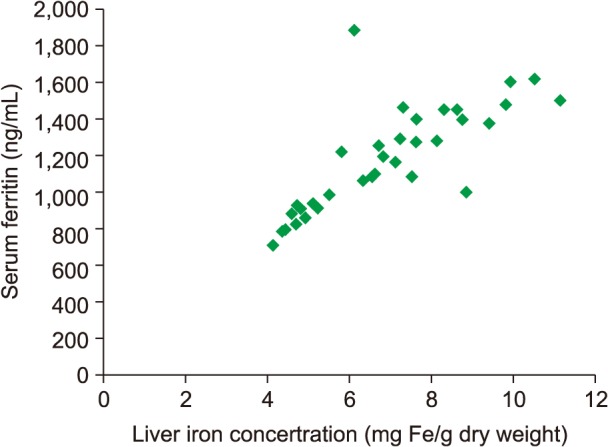
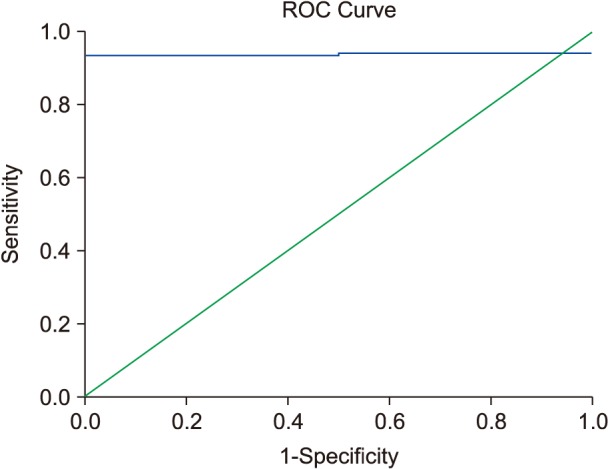
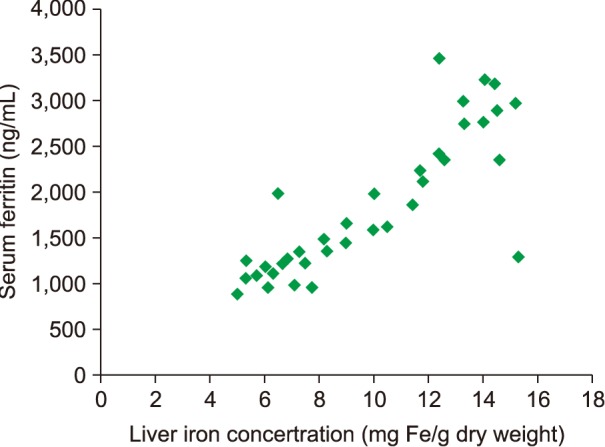
In patients with TI who had undergone a splenectomy (Fig. 2), we found a strong and significant correlation coefficient between SF levels and LIC readings (R=0.67; P<0.0001). SF levels >916 ng/mL were associated with LIC >5 mg Fe/g dry weight in ROC analysis (Fig. 3).
The mean SF for patients with TI with spontaneous and normal-onset puberty was significantly lower than for patients with delayed puberty [872 ng/mL (SD±715; range, 196–1634) vs. 1,221 ng/mL (SD±937; range, 356–3,201), P<0.0001]. In addition, the mean LIC reading in patients with TI who had spontaneous puberty was significantly lower than in patients with delayed onset of puberty [7.2 mg Fe/g dry weight (SD±5.3; range, 0.4–23.6) vs. 10.8 mg Fe/g dry weight (SD±4.6; range, 0.9–32.1), P<0.0001]. We found a significant and strong coefficient of correlation between SF levels and LIC readings (R=0.71; P<0.0001) in patients with TI who reached puberty spontaneously with no abnormal delay, as depicted in Fig. 4. SF level >940 ng/mL was associated with LIC >5 mg Fe/g dry weight in ROC analysis (Fig. 5).
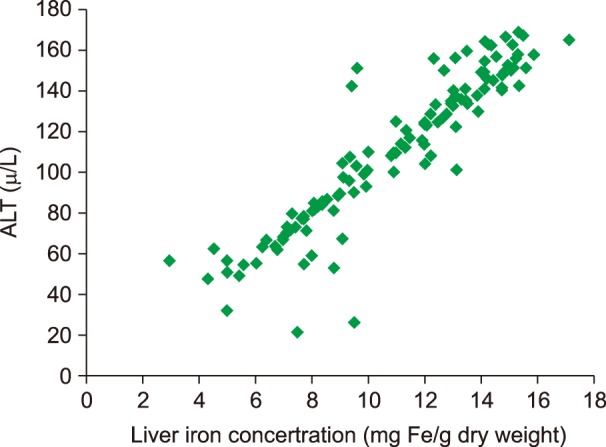
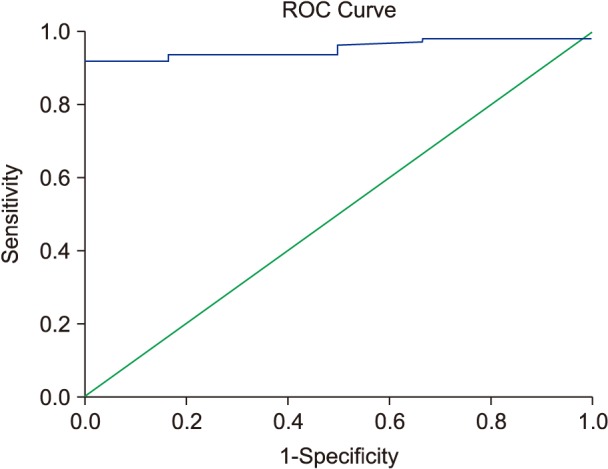
Liver enzymes (ALT) showed a highly significant coefficient of correlation with LIC in a subset of patients with TI (R=0.78; P<0.0001), depicted in Fig. 6. More than 3.15 times the upper normal limit of ALT level (63.5 vs. 20 U/L, which is the upper normal limit according to our laboratory) was correlated with LIC >5 mg Fe/g dry weight in ROC analysis (Fig. 7).
Go to : 
Without considering recent migration from East to West, the developing world has historically comprised the bulk of patients with thalassemia. Knowing when to chelate excess iron in patients with TI remains a challenge, owing to limited available resources for obtaining a clear diagnosis of iron overload, which may lead to increased morbidity and mortality [161718].
In Third World countries, tools other than SF levels for diagnosing iron overload are usually unavailable. It is widely known that SF is not a reliable diagnostic method in patients with TI, but some researchers have recognized SF >1,000 ng/mL as a cut-off for the use of iron-chelating drugs [9181920]. In this study, we sought to develop a collection of simple, inexpensive, easily available, and applicable criteria to obtain a clearer indication of body iron status in patients with TI, so as to administer iron chelation therapy at the appropriate time.
In our study, mean SF values in patients with TI were significantly lower than in patients with TM, despite comparable mean LIC values. These observations were also found by many authors, suggesting that in NTDT there is excessive absorption of iron, which is deposited within hepatocytes, leading to a decline in SF levels. In contrast, in transfusion-dependent thalassemia, iron is deposited mostly within reticuloendothelial tissues, where the synthesis and distribution of ferritin increase its serum levels [1921].
In patients with TI, LIC is 1 of the best indicators of iron overload, which is related to many complications, such as those in the skeletal, vascular, and endocrine systems. The higher the LIC levels, the more elevated the iron overload, until levels are reached that require the use of iron chelators [2223]. We did not find a significant direct correlation between LIC and SF. This has also been reported by other researchers [23], but different studies have reported differing results, which may suggest a wide range of iron accumulation levels among patients with TI [1819].
In patients with TI who have undergone splenectomy, our data showed a true level of significance for a direct positive correlation between LIC values and SF, which is in line with other previous data [18] but differs from additional studies in which no positive significance was observed [21], however; the small patient sample size might weaken the results.
Among our patients with TI who reached puberty spontaneously with no delay, a significant relationship between SF and LIC levels was found, with values of both that were lower than those in patients who had delayed puberty. The findings of other studies are inconsistent with our data, stressing the role of iron overload or hemosiderosis in delayed onset of puberty [1424].
ALT liver enzyme has been found to increase progressively with increasing LIC levels, showing a strong positive correlation. Limited published studies are available linking liver enzymes to LIC or true hemochromatosis in patients with TI, where normalization of these enzymes may be achieved with iron chelation therapy [25]. Other studies have linked elevated ALT and the presence of anti-hepatitis C virus antibodies to a high iron load in transfusion-dependent thalassemia [26].
Based on our data, which are in agreement with other previous studies, to prevent delay in the management of iron overload in patients with TI, iron chelation therapy may be started once LIC values surpass 5 mg Fe/g dry weight [1821222728]. Based on our sample of patients, we suggest that chelation therapy should be used when ALT levels increase to more than 3.15 times the upper normal laboratory limit and/or with SF levels above 916 and 940 ng/mL in patients with TI who have undergone splenectomy or those with spontaneous onset of puberty, respectively.
Our study may be the first work that links SF with LIC and that provides a cut-off limit in a special group of patients with TI (related to the spleen, puberty status, and/or liver enzyme values). A recent related study [29] proposed a general threshold of SF >800 ng/mL for starting iron chelation therapy in patients with TI, with no relation to any special criteria (such as splenectomy or puberty), using a value of ≥5 LIC mg Fe/g dry weight as a cut-off estimate, versus the value of >5 LIC mg Fe/g dry weight used in our study.
We hope that our findings will help to raise the interest of researchers for further investigation in studies that include a larger number of patients and are conducted in different parts of the world, to extend the understanding of other risk factors, such as frequency of blood transfusions, patient age, and chelation status (naïve or previously chelated). Such information will help in finding a low-cost, universal way to appropriately manage patients with TI, using simple methods that are widely available in developing countries where highly sophisticated tools (such as LIC) are lacking, to detect dangerous iron deposition in patients with TI.
There are some limitations in our study. First, SF was assessed using a single measurement taken on the same day as MRI examination; an average value of ferritin levels over the last 3–6 months might have more accurately reflected the true body iron status. Second, clinical criteria alone were used to rule out active infection/inflammation, without the use of any objective parameters such as estimation of C-reactive protein.
In conclusion, the findings of this study have essential implications for the management of patients with TI. Assessment of SF alone may result in delayed initiation of chelation therapy, which may prolong patient exposure to high iron levels and associated morbidity and mortality risks. Based on our results, and to prevent any delay in the management of iron overload in these patients, iron chelation therapy may be started with LIC values >5 mg Fe/g dry weight, ALT levels elevated to more than 3.15 times the upper normal laboratory limit, and/or SF levels above 916 and 940 ng/mL in patients with TI who have undergone splenectomy and those with spontaneous onset of puberty, respectively.
Go to : 
Notes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
Go to : 
References
1. Ben Salah N, Bou-Fakhredin R, Mellouli F, Taher AT. Revisiting beta thalassemia intermedia: past, present, and future prospects. Hematology. 2017; 22:607–616. PMID: 28589785.

2. Rivella S. β-thalassemias: paradigmatic diseases for scientific discoveries and development of innovative therapies. Haematologica. 2015; 100:418–430. PMID: 25828088.

3. Parrow NL, Gardenghi S, Ramos P, et al. Decreased hepcidin expression in murine β-thalassemia is associated with suppression of Bmp/Smad signaling. Blood. 2012; 119:3187–3189. PMID: 22461476.

4. Rivella S. The role of ineffective erythropoiesis in non- transfusion-dependent thalassemia. Blood Rev. 2012; 26(Suppl 1):S12–S15. PMID: 22631035.
5. Marsella M, Ammirabile M, Di Matola T, et al. Is there a difference in phenotype between males and females with nontransfusion-dependent thalassemia? A cross-sectional evaluation. Hematology. 2018; 23:522–525. PMID: 29303050.

6. Casu C, Aghajan M, Oikonomidou PR, Guo S, Monia BP, Rivella S. Combination of Tmprss6- ASO and the iron chelator deferiprone improves erythropoiesis and reduces iron overload in a mouse model of beta-thalassemia intermedia. Haematologica. 2016; 101:e8–e11. PMID: 26405152.

7. Fischer R, Harmatz PR. Non-invasive assessment of tissue iron overload. Hematology Am Soc Hematol Educ Program. 2009; 1:215–221.

8. Olivieri NF, Brittenham GM. Iron-chelating therapy and the treatment of thalassemia. Blood. 1997; 89:739–761. PMID: 9028304.

9. Porter JB, Elalfy M, Taher A, et al. Limitations of serum ferritin to predict liver iron concentration responses to deferasirox therapy in patients with transfusion-dependent thalassaemia. Eur J Haematol. 2017; 98:280–288. PMID: 27859648.

10. Olivieri NF, Brittenham GM, Matsui D, et al. Iron-chelation therapy with oral deferiprone in patients with thalassemia major. N Engl J Med. 1995; 332:918–922. PMID: 7877649.

11. Wood JC. Magnetic resonance imaging measurement of iron overload. Curr Opin Hematol. 2007; 14:183–190. PMID: 17414205.

12. Tanner JM. Growth at adolescence: with a general consideration of the effects of hereditary and environmental factors upon growth and maturation from birth to maturity. 2nd ed. Oxford, UK: Blackwell;1962.
13. Zachmann M, Prader A, Kind HP, Häfliger H, Budliger H. Testicular volume during adolescence. Cross-sectional and longitudinal studies. Helv Paediatr Acta. 1974; 29:61–72. PMID: 4838166.
14. Shalitin S, Carmi D, Weintrob N, et al. Serum ferritin level as a predictor of impaired growth and puberty in thalassemia major patients. Eur J Haematol. 2005; 74:93–100. PMID: 15654898.

15. Fung EB, Harmatz PR, Lee PD, et al. Increased prevalence of iron-overload associated endocrinopathy in thalassaemia versus sickle-cell disease. Br J Haematol. 2006; 135:574–582. PMID: 17054676.

16. Al-Momen H. Iron chelation therapy in sickle cell/beta thalassemia syndrome, a 2 years' extension study. KCMJ. 2017; 13:76–81.

17. Fung EB, Harmatz P, Milet M, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: a report from the multi-center study of iron overload. Am J Hematol. 2007; 82:255–265. PMID: 17094096.

18. Taher A, El Rassi F, Isma'eel H, Koussa S, Inati A, Cappellini MD. Correlation of liver iron concentration determined by R2 magnetic resonance imaging with serum ferritin in patients with thalassemia intermedia. Haematologica. 2008; 93:1584–1586. PMID: 18728025.

19. Pakbaz Z, Fischer R, Fung E, Nielsen P, Harmatz P, Vichinsky E. Serum ferritin underestimates liver iron concentration in transfusion independent thalassemia patients as compared to regularly transfused thalassemia and sickle cell patients. Pediatr Blood Cancer. 2007; 49:329–332. PMID: 17554789.

20. Fiorelli G, Fargion S, Piperno A, Battafarano N, Cappellini MD. Iron metabolism in thalassemia intermedia. Haematologica. 1990; 75:89–95. PMID: 2086386.
21. Origa R, Galanello R, Ganz T, et al. Liver iron concentrations and urinary hepcidin in beta-thalassemia. Haematologica. 2007; 92:583–588. PMID: 17488680.
22. Asadov C, Alimirzoeva Z, Mammadova T, Aliyeva G, Gafarova S, Mammadov J. β-thalassemia intermedia: a comprehensive overview and novel approaches. Int J Hematol. 2018; 108:5–21. PMID: 29380178.

23. El Rassi F, Cappellini MD, Inati A, Taher A. Beta-thalassemia intermedia: an overview. Pediatr Ann. 2008; 37:322–328. PMID: 18543543.

24. De Sanctis V. Growth and puberty and its management in thalassaemia. Horm Res. 2002; 58:72–79. PMID: 12373018.

25. Taher A, Isma'eel H, Cappellini MD. Thalassemia intermedia: revisited. Blood Cells Mol Dis. 2006; 37:12–20. PMID: 16737833.

26. Ameli M, Besharati S, Nemati K, Zamani F. Relationship between elevated liver enzyme with iron overload and viral hepatitis in thalassemia major patients in Northern Iran. Saudi Med J. 2008; 29:1611–1615. PMID: 18998011.
27. Majd Z, Haghpanah S, Ajami GH, et al. Serum ferritin levels correlation with heart and liver MRI and LIC in patients with transfusion-dependent thalassemia. Iran Red Crescent Med J. 2015; 17:e24959. PMID: 26023341.

28. Musallam KM, Cappellini MD, Taher AT. Evaluation of the 5mg/g liver iron concentration threshold and its association with morbidity in patients with β-thalassemia intermedia. Blood Cells Mol Dis. 2013; 51:35–38. PMID: 23425967.

29. Taher AT, Porter JB, Viprakasit V, et al. Defining serum ferritin thresholds to predict clinically relevant liver iron concentrations for guiding deferasirox therapy when MRI is unavailable in patients with non-transfusion-dependent thalassaemia. Br J Haematol. 2015; 168:284–290. PMID: 25212456.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download