Abstract
Coronary artery ectasia (CAE) is defined as the dilation of a segment of a coronary vessel to at least 1.5 times the diameter of its normal adjacent segment. Mean platelet volume (MPV) plays a role in acute coronary syndromes, with high MPV correlating to poor prognosis for acute thrombotic events and CAE. Several studies investigated the relationship between MPV and CAE, resulting in conflicting results. These results led us to systematically review all studies investigating the relationship between MPV and ectatic heart diseases by performing a meta-analysis study in order to report a unified result. This meta-analysis study investigated all case-control articles examining the relationship between MPV and CAE. All studies in the following databases published until January 31, 2018, were investigated: Science Direct, Scopus, PubMed, Google Scholar, and Web of Science. Following a quality control evaluation, 14 articles, all of which were published following studies performed in Turkey from 2007 to 2016, met the criteria for study inclusion. After pooling the results from all of the articles, a total standardized mean difference (SMD) value of 0.584 (95% CI, 0.219, 0.95) was obtained using the D+L pooled SMD, indicating a significant difference (P=0.002) between the two groups, with higher MPV values in ectatic patients when comparing to healthy individuals. Therefore, increased MPV levels were significantly related to CAE, suggesting that platelets, with their inflammatory and thrombotic activities, play a role in this disease. Therefore, anti-platelet and anti-inflammatory therapies may be effective in treating CAE.
Coronary heart disease is a principal disabling factor in industrialized societies and western countries. This disease results in disability and the loss of an individuals' productivity. Although significant medical improvements have been made with respect to the treatment of cardiac diseases, coronary heart disease is still one of the largest causes of death in western societies and in the world [12]. Also known as coronary artery aneurysm, coronary artery ectasia (CAE) is defined as the dilation of a segment of a coronary vessel at least 1.5 times the diameter of its normal adjacent segment. When dilation involves the whole vessel, it is called ectasia instead of aneurysmal disease [34].
CAE is a form of coronary atherosclerosis associated with risks of death and myocardial infarction. Currently, many more cases of people with CAE are being identified. The primary characteristics of CAE shown by angiography include impaired coronary flow, delayed coronary filling with contrast media, the reciprocating motion of contrast media (milking phenomenon), and retention of contrast media within dilated coronary segments [56].
According to the angiographic findings, 3% to 8% of patients with an atherosclerotic coronary artery suffered from ectasia [7]. Precise CAE pathogenesis has not been established. In 50% of cases, it has been attributed to atherosclerosis based on shared links, but it has not been supported by evidence currently provided through extensive lipidomic profiling. Up to 20% of CAE cases are considered congenital and 10% to 20% are associated with inflammatory diseases or connective tissue diseases such as Ehlers-Danlos syndrome, Kawasaki disease, and polycystic kidney disease. A coronary aneurysm is rarely seen after angioplasty or Takayesu arthritis (TA). There is some evidence indicating that CAE may be associated with inflammatory activities, but no consensus has been achieved on how to manage CAE [58].
Several factors are involved in causing coronary heart disease, with both genetic and environmental factors regarded as contributory factors. Many risk factors for coronary artery disease (CAD) have been reported, including hypertension, hyperlipidemia, increased age, male gender, CAD family history, ethnicity, smoking, lack of physical activity, emotional stress, obesity, metabolic syndrome, and insulin resistance. However, they are alone incapable of explaining the high prevalence of such diseases. Some people suffer from CAD without having known risk factors present, while others show these risk factors without having CAD. Further research is required to identify additional risk factors. As a result, some efforts are being made to search for new risk factors or biomarkers that could improve the prediction of cardiac diseases [910].
Platelets play a complex physiological role, being important to health in addition to illnesses due to contributions in hemostasis, inflammation, and tissue repair, as well as congenital and acquired immunity. In addition, they play an important role in the pathophysiology of cardiovascular thrombosis and inflammation. One major factor associated with CAD is platelet activation. Inflammatory mediators such as interleukins 1, 3, and 6 increase megakaryocyte proliferation increasing the number of platelets. Increased platelet numbers can be a sign of inflammation and along with activating thrombocytes can create a thrombosis-favoring environment.
Platelets with dense granules are larger and more metabolically active. Effective markers of CAD are mean platelet volume (MPV), platelet activation, uric acid, and gamma-glutamyl transferase (GGT) blood levels, all of which are valuable predictors for serious outcomes of acute coronary syndromes [111213].
MPV is the mean volume of circulating platelets in femtoliters and suggests their function and activation, and they play an important role in coronary heart disease pathophysiology. Increased MPV is metabolically and enzymatically associated with more active platelets and has a higher effect on hemostasis compared to smaller, less active ones [1415]. Increased MPV may be due to the production of more precursors of larger platelets, in vivo swollen platelets, and/or degeneration of smaller platelets; however, its mechanisms have not been clearly determined. Increased MPV is associated with acute coronary syndrome, carotid artery disease, deep venous thrombosis, slow coronary circulation phenomenon, and coronary pulmonary and collateral vessel embolism [11].
Many recent studies have investigated the relationship between MPV and CAD. Based on performed studies, MPV is involved in acute coronary syndromes and high levels of MPV relate to poor prognosis for acute thrombotic events. Additionally, atherosclerosis and cardiovascular risk factors are frequently associated with CAE [15].
Several studies have investigated the relationship between MPV and CAE, obtaining no clear or precise results. Investigating the results from a combination of 40 studies performed around the world, a meta-analysis revealed that the levels of MPV are higher in patients with cardiac diseases than in healthy people [9]. However, there is some disagreement in regard to ectatic diseases, where higher levels of MPV were shown for patients with cardiac diseases in some studies when comparing to healthy individuals, while the opposite was shown in other studies. Among these studies, Keser et al. [1617] observed no significant differences between a group of ectatic patients and a group of healthy people with respect to the levels of MPV. However, Sarli et al. [1218] showed that there were significant differences between the two groups.
There has been a significant amount of research examining the role of MPV as a possible risk factor for ectatic heart disease, with discordant results. Strong, weak, and no relationship between MPV and ectasia have been reported. These conflicting results led us to systematically review all studies investigating the relationship between MPV and ectatic heart diseases by performing a meta-analysis study in order to give an overall uniform result.
This meta-analysis study investigated all case-control articles examining the relationships between MPV and CAE. All studies in the following databases published until January 31, 2018, were investigated: Science Direct, Scopus, PubMed, Google Scholar, and Web of Science. The mechanism of searching for articles was largely implemented using a structured search for keywords, with all possible important combinations (mean platelet volumes or platelet volumes or inflammatory markers and coronary artery ectasia or coronary disease). In addition, we examine all references of articles published in this field.
In the present study, two researchers performed the search process independently. All articles related to the study objectives were compiled by checking the subject and/or abstract of the article, while unrelated ones were excluded. Required information was collected from relevant articles and their abstracts.
1) Case-control studies reporting mean values and standard deviation for MPV for both case and control groups.
2) In situations where the findings of an article were published under two different subjects by two different journals, one would be used which was published by the valid one.
Ecological, cross-sectional, and case-control studies which did not report mean values and standard deviation values of MPV and/or such values of which were impossible to calculate were excluded from the study.
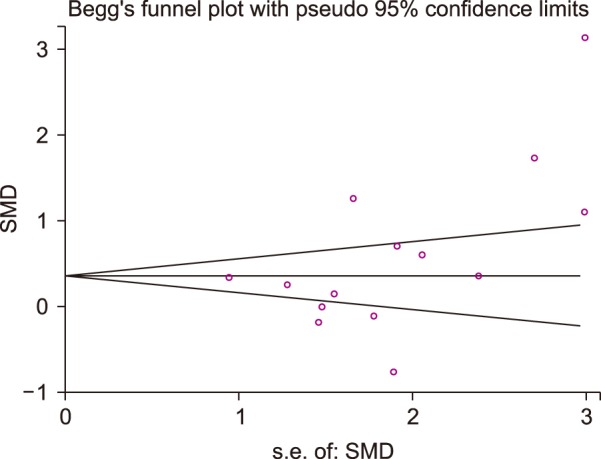
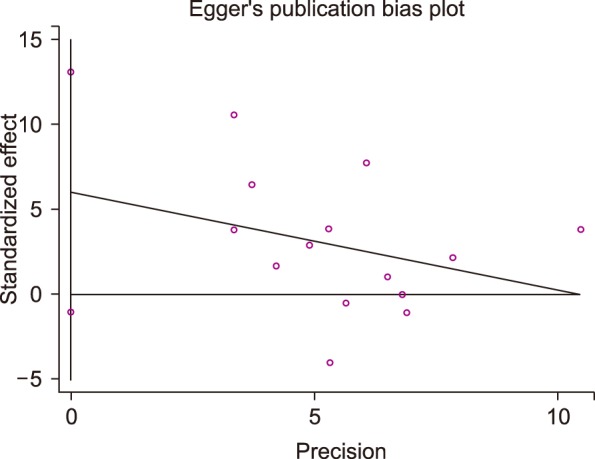
After performing the final evaluation of the quality of the articles, key information was obtained from a pre-prepared checklist. Such information included the numbers of subjects in both case and control groups, mean age and gender of the subjects, mean and standard deviation values of MPV, the last name of responsible authors, year of publishing, and the place where each study was performed. Extracted data were entered into a Microsoft Excel spreadsheet and the data were analyzed by Stata 12 software. Given the results from heterogeneous studies using Cochran's Q test and the Higgins-Thompson's I2 transformation, two fixed and random effect models were used for continuous data at the 95% confidence level in order for the data to be analyzed. For continuous outcomes, the mean MPV difference between CAE and control groups were estimated for each study and pooled using a standardized mean difference. Funnel plots were used to evaluate publication bias, the symmetry of which was examined by Egger's and Begg's statistical tests.
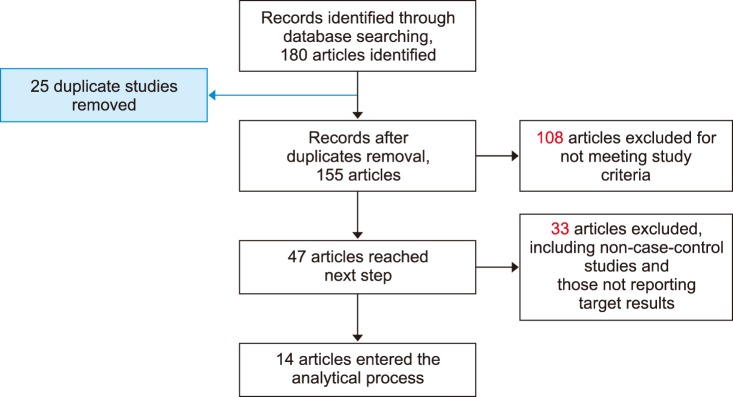
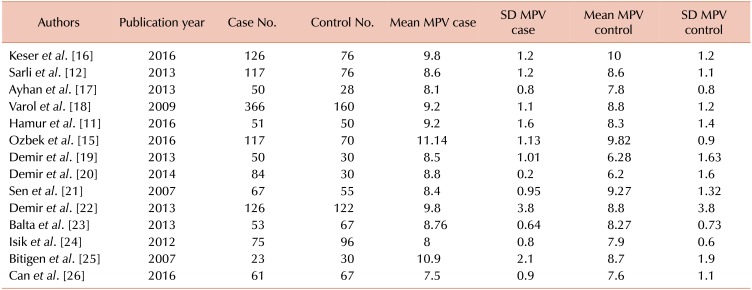
A total of 14 articles met the inclusion and quality control criteria for this study, all of which were published from studies performed in Turkey from 2007 to 2016 (Fig. 1) [1112151617181920212223242526]. A total of 2,323 people were evaluated in the mentioned studies, including 957 healthy individuals [465 females (F) and 492 males (M)] with mean ages of 55±5.25 (for F) and 37±5.25 (for M) years and 1,366 patients [610 females (F) and 748 males (M)] with mean ages for females of 58±4.3 years and 58±4.3 years for males (Table 1).
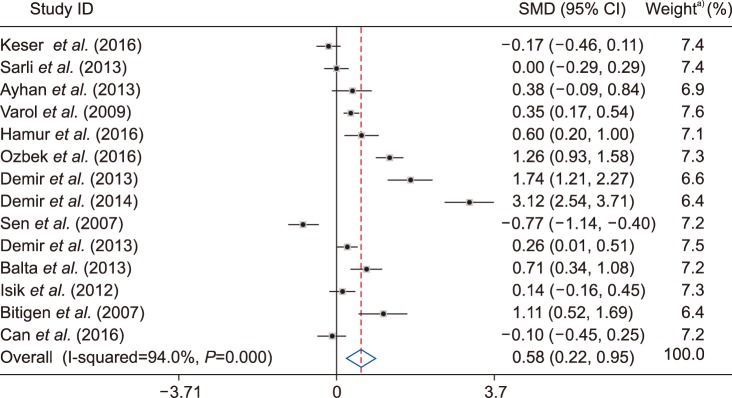
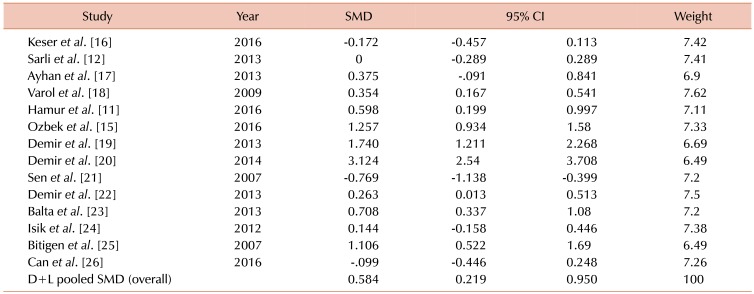
Among the 14 studies investigated, the studies by Sen et al. (2007) (95% CI, −1.138 to −0.399) and Demir et al. (2014) (95% CI, 2.54 to 3.708) showed the lowest and highest values of MPV SMD of −0.769 and 3.124, respectively [2021]. After pooling the results of all articles, a total SMD value of 0.584 (95% CI, 0.219, 0.95) was obtained using the D+L pooled SMD, indicating a significant difference (P=0.002) between the two groups and higher MPV values in the ectatic patient group in comparison with healthy individuals (Table 2).
Considering the results reported for case and control groups, values of MPV mean and standard deviation were extracted from the various studies mentioned. Fixed and random effect models were employed. Two indices, including Cochran' Q and I2 statistics, were used to examine heterogeneity among studies. Heterogeneity tests were administered to test the null hypothesis that groups had similar MPV values. A heterogeneity effect was determined using I2, which was conflicting among different studies and determined that internal study variations were due to heterogeneity rather than by chance. Values of I2 greater than 50 indicated the presence of heterogeneity among studies. The present study employed the random effects method and Der Simonian & Laird method, producing values of I2=94% and P<0.001. A value of SMD was obtained at 0.58, indicating that the mean value of MPV was higher in the patient group than in the normal, control group (P=0.002) (Fig. 2).
Using a meta-analysis method, we examined 14 case-control studies investigating the relationship between MPV and CAE. This is the first study which examined mean differences of MPV between individuals with CAE and healthy people. Our results showed a higher value of MPV SMD (SMD=0.584 fL) for people with CAE compared to healthy controls (95% CI, 0.219, 0.95).
In a meta-analysis study examining mean differences (MD) between MPV and CAD, Sansanayudh et al. [9] obtained results similar to ours, that is, SMD values of MPV were significantly higher for CAD patients than those for healthy individuals (unstandardized SMD of 0.70 fL; 95% CI, 0.55, 0.85).
Increased MPV can be associated with the production of more precursors of larger platelets, in vivo swollen platelets, and/or degeneration of smaller platelets. However, its precise mechanisms remain to be determined. Different cardiovascular diseases such as acute myocardial infarction, acute ischemic stroke, preeclampsia, and acute renal stenosis have exhibited increased MPV levels. High MPV levels are also associated with poor prognosis in acute thrombotic events. Atherosclerosis and cardiovascular risk factors are also frequently associated with CAE [1521].
Increasing platelet volume in parallel with increasing enzymatic and metabolic activities result in an increase in thromboxane A2 and B2 relative to the volume and an increase in glycoprotein IIb-IIa emergence. Platelet accumulation is increased by adenosine diphosphate and decreased by prostacyclin. Active platelets are larger and denser, containing alpha granules which themselves contain platelet factor 4, P-selectin, and platelet-derived growth factor, which act as mitogenic factors in neointimal proliferation and cause inflammatory cell adhesion, platelet accumulation, and thrombosis [22].
Not surprisingly, theories on the origin of ectasia relate to the vascular endothelium and biological characteristics of arterial walls although coronary ectatic disease shows similar histopathological characteristics to that of coronary atherosclerosis. However, the specific mechanism causing abnormal dilation of ectatic vessels is unknown. Histologically, ectatic segments show vast atherosclerotic changes and degeneration of the media layer of vascular walls. More than 50% of patients with ectasia show comorbidity with coronary atherosclerosis disease, suggesting that coronary ectasia is a form of CAD, but some collagenous, connective tissue, and vasculitis disease also exits. Coronary ectasia disease increases patient disability and mortality rates through mechanisms that include slow coronary flow, coronary vasospasm, dissection, and thrombus formation [21].
Varol et al. found results similar to ours in their study aimed at assessing MPV in patients with CAE (N=366) and compared patients with a healthy (control) group (N=160). They obtained significantly higher MPV values for the case group with CAE than those for the control group (P<0.001; 9.2±1.1 fL vs. 8.8±1.2 fL, respectively). They also specified that the number of platelets in CAE patients was lower than that of controls, as opposed to the results of prior studies where authors found no significant differences in the number of platelets between ectatic groups and controls. MPV is inversely related to the number of platelets, which was suggested to be due to consumption of small platelets and compensatory production of large ones.
Intravascular thrombosis of ectatic vessel segments results in platelet consumption and their reduced number, and in turn, stimulates the bone marrow to produce larger platelets. Such increased MPV, which reflects activity variations and accumulation of platelets, is observed in patients only with coronary ectasia. As a result, microvascular disorders and increased activity of platelets can lead to thrombus formation in ectatic patients. Elevated MPV levels suggest that, due to increased platelet activity, ectatic patients are at a high risk for acute coronary events. In this regard, studies place an emphasis on anti-platelet treatments [18].
With the aim of assessing platelet volume indices in patients with CAE, Hamur et al. [11] (2016) performed a study consisting of 51 patients with CAE and 50 healthy controls, which was a smaller study in comparison with that of the study performed by Varol et al. [18], where MPV values were significantly higher in the CAE group than those in the control group (P<0.05; 9.2 vs. 8.3). However, in the Hamur et al. study results from multivariate analyses reported no significant relationship between MPV values and CAE [odds ratio for MPV (0.318–2.336 fL); 95% CI; P=0.769, 0.861] [1118].
Although many studies identified GGT, uric acid, and MPV as being associated with CAE, their sample size was so small that definitive relationships between these markers and CAE could not be extrapolated. Therefore, extant evidence of relationships between CAE and these markers in weak [12].
Thrombotic particles inside ectatic end segments of coronary vessels which are embolized because of variations in blood viscosity, activation of the coagulation system, platelet activation, and circulation shift from laminar to turbulent flow within ectatic segments are responsible for causing ischemia and infarction in CAE cases. Reduced velocity and changed patterns of coronary blood flow within dilated segments, as with variations in platelet activity can accelerate thrombus formation [25].
MPV helps to differentiate hyperdestructive thrombocytopenia such as idiopathic thrombocytopenic purpura from hypo-productive thrombocytopenia because its levels are higher in the former than that of the latter [27].
Ozbek et al. [15] (2016) investigated the relationship between MPV and severity of CAE by performing a case-control study on 117 patients and 70 healthy controls. Results of their study indicated that MPV values were significantly higher in the CAE group than those in the control group (P<0.001; 11.14±1.13 fL vs 9.82±0.90 fL). In addition, results from multiple logistic regression analysis (P<0.01; OR=3.555; 95% CI; MPV, 2.282–5.538) indicated that MPV acts as an independent predictor of CAE, increasing the chance of affliction by 3.5 times.
Ozbek et al. [15] concluded that both MPV and red blood cell distribution width (RDW) increased with CAE and MPV and were positively correlated. High MPV was related to CAE severity. For example, MPV greater than 10.85 fL reflected the presence and severity of CAE and high MPV levels indicated the necessity of a more invasive anti-platelet treatment for CAE patients.
Increased MPV is in parallel with shorter bleeding time and elevated plasma levels of thromboxane B2. High MPV values are a reliable marker of increased platelet activity. Larger platelets have more stored granules and receptors and adhere more rapidly compared to smaller platelets. Thus, platelets activity is represented more accurately by their larger size rather than their count [2829].
Based on the findings of the present research, increased levels of MPV are significantly related to CAE, suggesting that platelets as well as inflammatory and thrombotic activities, play a role in this disease. Additionally, anti-platelet and anti-inflammatory treatments may be effective in treating CAE.
ACKNOWLEDGMENTS
The authors would like to thank the personnel and experts from the Clinical Research Development Center, Imam Ali and Taleghani Hospitals and Kermanshah University of Medical Sciences for their assistance in obtaining the required articles.
References
1. Beresniak A, Caruba T, Sabatier B, Juillière Y, Dubourg O, Danchin N. Cost-effectiveness modelling of percutaneous coronary interventions in stable coronary artery disease. World J Cardiol. 2015; 7:594–602. PMID: 26516413.

2. Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015; 128:229–238. PMID: 25447615.

3. Yu L, Gu T, Shi E, Xiu Z, Fang Q, Liu B. Surgical repair of coronary artery fistula combined with coronary artery ectasia in adults. J Card Surg. 2013; 28:222–227. PMID: 23675679.

4. Zipes D, Libby P, Bonow RO, Mann DL, Tomaselli GF. Braunwald's heart disease: a textbook of cardiovascular medicine. 11th ed. Philadelphia, PA: Elsevier;2018. p. 63.
5. Wang Y, Wang Y, Yin D, Dou K, Wu Y, Song W. The beneficial effects of renin-angiotensin system blockades on 2 year outcomes in coronary artery ectasia patients. Curr Med Res Opin. 2017; 33:1677–1684. PMID: 28598221.

6. Arif Yalcin A, Faruk Akturk I, Celik O, et al. Coronary artery ectasia is associated with the c.894G>T (Glu298Asp) polymorphism of the endothelial nitric oxide synthase gene. Tohoku J Exp Med. 2014; 232:137–144. PMID: 24573064.
7. Fariba F, Moradi M, Arabi A, Ghaderi B. Prevalence of coronary artery ectasia with atherosclerosis and associated risk factors in the West of Iran: a cross-sectional study. J Res Health Sci. 2016; 16:22–25. PMID: 27061992.
8. Boles U, Johansson A, Wiklund U, et al. Cytokine disturbances in coronary artery ectasia do not support atherosclerosis pathogenesis. Int J Mol Sci. 2018; 19:E260. PMID: 29337902.

9. Sansanayudh N, Anothaisintawee T, Muntham D, McEvoy M, Attia J, Thakkinstian A. Mean platelet volume and coronary artery disease: a systematic review and meta-analysis. Int J Cardiol. 2014; 175:433–440. PMID: 25017904.

10. Sharma V, Aggarwal A. Helicobacter pylori: Does it add to risk of coronary artery disease. World J Cardiol. 2015; 7:19–25. PMID: 25632315.

11. Hamur H, Kalkan K, Duman H, et al. Plateletcrit and platelet distribution width as independent predictors of coronary artery ectasia. Koşuyolu Heart J. 2016; 19:173–178.

12. Sarli B, Baktır AO, Saglam H, et al. No relevant association between coronary artery ectasia and mean platelet volume, gamma-glutamyltransferase and uric acid levels. Turk Kardiyol Dern Ars. 2013; 41:598–603. PMID: 24164990.
13. Soydinc S, Turkbeyler IH, Pehlivan Y, et al. Mean platelet volume seems to be a valuable marker in patients with systemic sclerosis. Inflammation. 2014; 37:100–106. PMID: 23996103.

14. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011; 17:47–58. PMID: 21247392.
15. Ozbek K, Katlandur H, Keser A, Ulucan S, Ozdil H, Ulgen MS. Is there a relationship between mean platelet volume and the severity of coronary ectasia? Biomed Res. 2016; 27:816–820.
16. Keser A, Ozbek K, Ulucan S, Katlandur H, Bilgi M, Ozdil H. Relationship between red cell distribution width levels and severity of coronary artery ectasia. Eur Rev Med Pharmacol Sci. 2016; 20:1571–1574. PMID: 27160130.
17. Ayhan SS, Ozturk S, Erdem A, et al. Relation of neutrophil/lymphocyte ratio with the presence and severity of coronary artery ectasia. Turk Kardiyol Dern Ars. 2013; 41:185–190. PMID: 23703551.
18. Varol E, Akcay S, Ozaydin M, Erdogan D, Dogan A. Mean platelet volume in patients with coronary artery ectasia. Blood Coagul Fibrinolysis. 2009; 20:321–324. PMID: 19417633.

19. Demir M, Keceoglu S, Melek M. The relationship between blood monocyte count and coronary artery ectasia. Cardiol Res. 2013; 4:159–164. PMID: 28352439.

20. Demir M, Demir C, Keceoglu S. The relationship between blood monocyte count and ccoronary artery ectasia. Cardiol Res. 2014; 5:151–154. PMID: 28348713.
21. Sen N, Tavil Y, Yazici HU, et al. Mean platelet volume in patients with coronary artery ectasia. Med Sci Monit. 2007; 13:CR356–CR359. PMID: 17660725.
22. Demir S, Avsar MK, Karakaya Z, et al. Increased mean platelet volume is associated with coronary artery ectasia. Postępy Kardiol Interwencyjnej. 2013; 9:241–245. PMID: 24570725.

23. Balta S, Demirkol S, Celik T, et al. Association between coronary artery ectasia and neutrophil-lymphocyte ratio. Angiology. 2013; 64:627–632. PMID: 23471489.

24. Isik T, Kurt M, Ayhan E, et al. Relation of red cell distribution width with presence and severity of coronary artery ectasia. Clin Appl Thromb Hemost. 2012; 18:441–447. PMID: 22619398.

25. Bitigen A, Tanalp AC, Elonu OH, Karavelioglu Y, Ozdemir N. Mean platelet volume in patients with isolated coronary artery ectasia. J Thromb Thrombolysis. 2007; 24:99–103. PMID: 17243013.

26. Can Y, Harun K, Kocayigit I, et al. Association between coronary artery ectasia and platelet to lymphocyte ratio. Am J Cardiol. 2016; 117(Suppl 1):S46.
27. Erdem E, Erdem D, Dilek M, et al. Red cell distribution width and mean platelet volume in amyloidosis. Clin Appl Thromb Hemost. 2014; 20:334–337. PMID: 23076775.

28. Beyan C, Beyan E. Mean platelet volume may not be changed in patients with coronary artery ectasia. Erciyes Med J. 2015; 37:85–86.

29. Yilmaz F, Koklu E, Kizilirmak Yilmaz F, Sarionder Gencer E, Alparslan AS, Yildırimturk O. Evaluation of mean platelet volume and platelet distribution width in patients with asymptomatic intermediate carotid artery plaque. Kardiol Pol. 2017; 75:35–41. PMID: 27714714.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download