Abstract
Pregnancy rhinitis is a relatively common condition. It is characterized by the presence of nasal symptoms, especially nasal congestion, not present prior to pregnancy, but typically present during the last 6 or more weeks of pregnancy, without other signs of respiratory tract infection or any known allergic causes, and disappearing completely within 2 weeks after delivery. Nasal saline irrigation, intranasal steroid spray, and oral antihistamines are usually recommended as the first line of treatment for rhinitis. However, most pregnant women refuse medical treatment for pregnancy rhinitis because of the fear of teratogenicity. Severe pregnancy rhinitis increases the risk of snoring, which has been suggested as having adverse effects on the fetus. In cases where the patients are unable to control their symptoms, pregnancy rhinitis can negatively affect the quality of life (QOL) as well as the pregnancy outcome. Therefore, special caution is required for determining the appropriate diagnosis and treatment modalities for pregnancy rhinitis. Here, we report for the first time, the successful treatment of pregnancy rhinitis that was unresponsive to conservative management and medical therapy by using microdebrider-assisted inferior turbinoplasty at the final stages of pregnancy, along with a review of the relevant literature.
REFERENCES
1). Ellegård EK. The etiology and management of pregnancy rhinitis. Am J Respir Med. 2003. 2:469–75.

2). Kumar R., Hayhurst KL., Robson AK. Ear, nose, and throat manifestations during pregnancy. Otolaryngol Head Neck Surg. 2011. 145:188–98.

3). Ellegård EK. Clinical and pathogenetic characteristics of pregnancy rhinitis. Clin Rev Allergy Immunol. 2004. 26:149–59.

4). Vlastarakos PV., Manolopoulos L., Ferekidis E., Antsaklis A., Niko-lopoulos TP. Treating common problems of the nose and throat in pregnancy: what is safe? Eur Arch Otorhinolaryngol. 2008. 265:499–508.

5). Settipane RA. Other causes of rhinitis: mixed rhinitis, rhinitis medi-camentosa, hormonal rhinitis, rhinitis of the elderly, and gustatory rhinitis. Immunol Allergy Clin North Am. 2011. 31:457–67.
6). Gilbey P., McGruthers L., Morency AM., Shrim A. Rhinosinusitis-related quality of life during pregnancy. Am J Rhinol Allergy. 2012. 26:283–6.

8). Goldstein G., Govindaraj S. Rhinologic issue in pregnancy. Allergy Rhinol (Providence). 2012. 3:e13–5.
9). Choi JH., Jun YJ., Oh JI., Jung JY., Hwang GH., Yum GH, et al. Impact of open-mouth breathing on upper airway anatomy in patients with sleep-disordered breathing. J Rhinol. 2012. 19:55–9.
10). Lee SK., Kim SW., Lee YC., Kim SH., Eun YG., Cho JS. Difference of polysomnographic, anthropometric, physical and radiologic findings in an obstructive sleep apnea syndrome patient with excessive daytime sleepiness. J Rhinol. 2008. 15:55–61.
11). Ellegård EK. Special consideration in the treatment of pregnancy rhinitis. Womens Health (Lond). 2005. 1:105–14.
12). Romano A., Orabona GD., Salzano G., Abbate V., Iaconetta G., Califa-no L. Comparative study between partial inferior turbinotomy and microdebrider-assisted inferior turbinoplasty. J Craniofac Surg. 2015. 26:e235–8.

13). Yau WP., Mitchell AA., Lin KJ., Werler MM., Hernández-Díaz S. Use of decongestants during pregnancy and the risk of birth defects. Am J Epidemiol. 2013. 178:198–208.

14). Rocklin RE. Asthma, asthma medications and their effects on ma-ternal/fetal outcomes during pregnancy. Reprod Toxicol. 2011. 32:189–97.

15). Incaudo GA., Takach P. The diagnosis and treatment of allergic rhinitis during pregnancy and lactation. Immunol Allergy Clin North Am. 2006. 26:137–54.

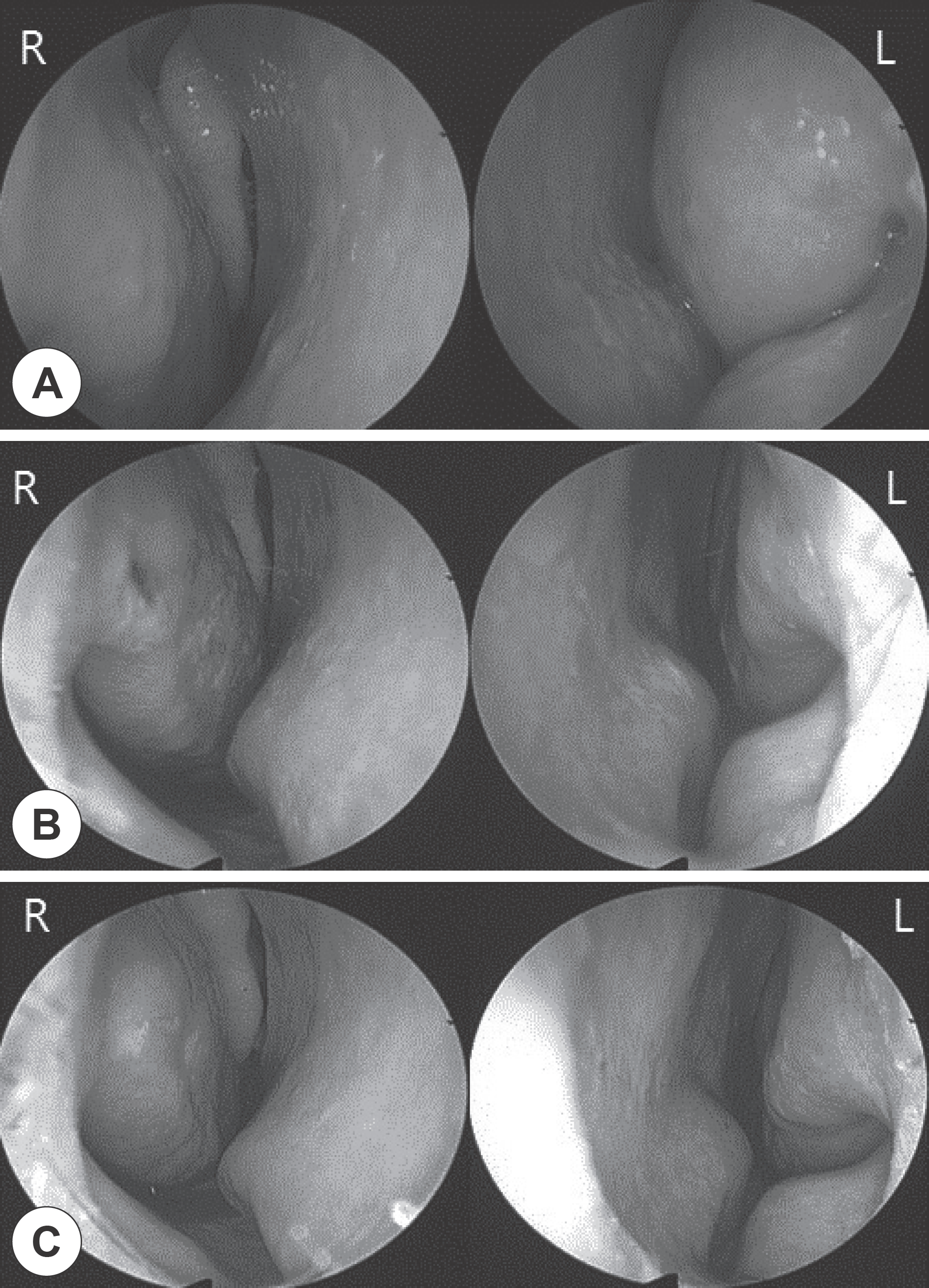
Fig. 1
Endoscopic views of the inferior turbinate. A: Preoperative. B: Postoperative, 1 week. C: Postoperative, 4 weeks (R, Right; L,: Left).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download