1. Klugman KP, Black S, Dagan R, Malley R, Whitney CG. Pneumococcal vaccine. In : Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Philadelphia, PA: Elsevier Health Sciences;2013. p. 504–541.
2. Fedson DS, Scott JA. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine. 1999; 17(Suppl 1):S11–S18.

3. Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009; 16(3):376–381. PMID:
19144787.

4. Kyaw MH, Jones IG, Campbell H. Prevention of pneumococcal disease in children. Pneumococcal conjugate vaccines: their use globally could have a major impact on public health. Acta Paediatr. 2001; 90(5):473–476. PMID:
11430703.

5. Ortqvist A. Pneumococcal vaccination: current and future issues. Eur Respir J. 2001; 18(1):184–195. PMID:
11510792.
6. Peters TR, Edwards KM. Pneumococcal vaccines: present and future. Pediatr Ann. 2002; 31(4):261–268. PMID:
11966249.

7. Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome CV, Facklam RR. Pneumococcal polysaccharide vaccine efficacy. An evaluation of current recommendations. JAMA. 1993; 270(15):1826–1831. PMID:
8411526.

8. Puumalainen T, Ekström N, Zeta-Capeding R, Ollgren J, Jousimies K, Lucero M, et al. Functional antibodies elicited by an 11-valent diphtheria-tetanus toxoid-conjugated pneumococcal vaccine. J Infect Dis. 2003; 187(11):1704–1708. PMID:
12751027.

9. Rennels MB, Edwards KM, Keyserling HL, Reisinger KS, Hogerman DA, Madore DV, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998; 101(4 Pt 1):604–611. PMID:
9521941.

10. Shinefield HR, Black S, Ray P, Chang I, Lewis N, Fireman B, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999; 18(9):757–763. PMID:
10493334.

11. Shinefield HR, Black S. Efficacy of pneumococcal conjugate vaccines in large scale field trials. Pediatr Infect Dis J. 2000; 19(4):394–397. PMID:
10783042.

12. Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003; 348(18):1737–1746. PMID:
12724479.

13. Esposito S, Tansey S, Thompson A, Razmpour A, Liang J, Jones TR, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol. 2010; 17(6):1017–1026. PMID:
20427630.

14. Chevallier B, Vesikari T, Brzostek J, Knuf M, Bermal N, Aristegui J, et al. Safety and reactogenicity of the 10-valent pneumococcal non-typeable
Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. Pediatr Infect Dis J. 2009; 28(4):Suppl. S109–S118. PMID:
19325447.
15. Harboe ZB, Benfield TL, Valentiner-Branth P, Hjuler T, Lambertsen L, Kaltoft M, et al. Temporal trends in invasive pneumococcal disease and pneumococcal serotypes over 7 decades. Clin Infect Dis. 2010; 50(3):329–337. PMID:
20047478.

16. Navarro Torné A, Dias JG, Quinten C, Hruba F, Busana MC, Lopalco PL, et al. European enhanced surveillance of invasive pneumococcal disease in 2010: data from 26 European countries in the post-heptavalent conjugate vaccine era. Vaccine. 2014; 32(29):3644–3650. PMID:
24795228.

17. Mendes RE, Costello AJ, Jacobs MR, Biek D, Critchley IA, Jones RN. Serotype distribution and antimicrobial susceptibility of USA
Streptococcus pneumoniae isolates collected prior to and post introduction of 13-valent pneumococcal conjugate vaccine. Diagn Microbiol Infect Dis. 2014; 80(1):19–25. PMID:
24974272.
18. Rosen JB, Thomas AR, Lexau CA, Reingold A, Hadler JL, Harrison LH, et al. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis. 2011; 53(2):137–143. PMID:
21690620.

19. O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, et al. Burden of disease caused by
Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009; 374(9693):893–902. PMID:
19748398.
20. Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015; 15(5):535–543. PMID:
25801458.

21. van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, et al. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014; 32(34):4349–4355. PMID:
24657717.

22. Kawaguchiya M, Urushibara N, Aung MS, Morimoto S, Ito M, Kudo K, et al. Emerging non-PCV13 serotypes of noninvasive
Streptococcus pneumoniae with macrolide resistance genes in northern Japan. New Microbes New Infect. 2015; 9:66–72. PMID:
26909157.
23. Ben-Shimol S, Greenberg D, Givon-Lavi N, Schlesinger Y, Somekh E, Aviner S, et al. Early impact of sequential introduction of 7-valent and 13-valent pneumococcal conjugate vaccine on IPD in Israeli children <5 years: an active prospective nationwide surveillance. Vaccine. 2014; 32(27):3452–3459. PMID:
24690148.
24. Golden AR, Adam HJ, Gilmour MW, Baxter MR, Martin I, Nichol KA, et al. Assessment of multidrug resistance, clonality and virulence in non-PCV-13
Streptococcus pneumoniae serotypes in Canada, 2011–13. J Antimicrob Chemother. 2015; 70(7):1960–1964. PMID:
25761605.
25. Varon E, Cohen R, Béchet S, Doit C, Levy C. Invasive disease potential of pneumococci before and after the 13-valent pneumococcal conjugate vaccine implementation in children. Vaccine. 2015; 33(46):6178–6185. PMID:
26476365.

26. Skinner JM, Indrawati L, Cannon J, Blue J, Winters M, Macnair J, et al. Pre-clinical evaluation of a 15-valent pneumococcal conjugate vaccine (PCV15-CRM197) in an infant-rhesus monkey immunogenicity model. Vaccine. 2011; 29(48):8870–8876. PMID:
21964055.

27. Jódar L, Butler J, Carlone G, Dagan R, Goldblatt D, Käyhty H, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003; 21(23):3265–3272. PMID:
12804857.

28. Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006; 13(2):165–169. PMID:
16467321.

29. Rubins JB, Alter M, Loch J, Janoff EN. Determination of antibody responses of elderly adults to all 23 capsular polysaccharides after pneumococcal vaccination. Infect Immun. 1999; 67(11):5979–5984. PMID:
10531257.

30. Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006; 13(9):1004–1009. PMID:
16960111.

31. Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979; 54(3):713–733. PMID:
288488.

32. Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, et al. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against
Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997; 4(4):415–422. PMID:
9220157.
33. Fleck RA, Romero-Steiner S, Nahm MH. Use of HL-60 cell line to measure opsonic capacity of pneumococcal antibodies. Clin Diagn Lab Immunol. 2005; 12(1):19–27. PMID:
15642980.

34. Burton RL, Nahm MH. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in
Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol. 2012; 19(6):835–841. PMID:
22518015.
35. Calix JJ, Porambo RJ, Brady AM, Larson TR, Yother J, Abeygunwardana C, et al. Biochemical, genetic, and serological characterization of two capsule subtypes among
Streptococcus pneumoniae serotype 20 strains: discovery of a new pneumococcal serotype. J Biol Chem. 2012; 287(33):27885–27894. PMID:
22736767.
36. Findlay JW, Smith WC, Lee JW, Nordblom GD, Das I, DeSilva BS, et al. Validation of immunoassays for bioanalysis: a pharmaceutical industry perspective. J Pharm Biomed Anal. 2000; 21(6):1249–1273. PMID:
10708409.

37. Henckaerts I, Durant N, De Grave D, Schuerman L, Poolman J. Validation of a routine opsonophagocytosis assay to predict invasive pneumococcal disease efficacy of conjugate vaccine in children. Vaccine. 2007; 25(13):2518–2527. PMID:
17034907.

38. Gijzen K, Liu WM, Visontai I, Oftung F, van der Werf S, Korsvold GE, et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine. 2010; 28(19):3416–3422. PMID:
20206285.

39. Li G, Liang Q, Shi J, Hu Y, Li H, Wei W, et al. Safety and immunogenicity of 23-valent pneumococcal polysaccharide vaccine in 2 to 70 year old healthy people in China: a phase III double blind, randomized clinical trial. Hum Vaccin Immunother. 2015; 11(3):699–703. PMID:
25714798.

40. Lee S, Kim HW, Lee JH, Kim KH. Functional immune responses to 11 non-PCV13 serotypes after immunization with a 23-valent pneumococcal polysaccharide vaccine in older adults. Vaccine. 2017; 35(37):4960–4965. PMID:
28778614.

41. Pilishvili T, Lexau C, Farley MM, Hadler J, Harrison LH, Bennett NM, et al. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010; 201(1):32–41. PMID:
19947881.

42. McFetridge R, Meulen AS, Folkerth SD, Hoekstra JA, Dallas M, Hoover PA, et al. Safety, tolerability, and immunogenicity of 15-valent pneumococcal conjugate vaccine in healthy adults. Vaccine. 2015; 33(24):2793–2799. PMID:
25913828.

43. Burton RL, Antonello J, Cooper D, Goldblatt D, Kim KH, Plikaytis BD, et al. Assignment of opsonic values to pneumococcal reference serum 007sp for use in opsonophagocytic assays for 13 serotypes. Clin Vaccine Immunol. 2017; 24(2):e00457–00516. PMID:
27974397.


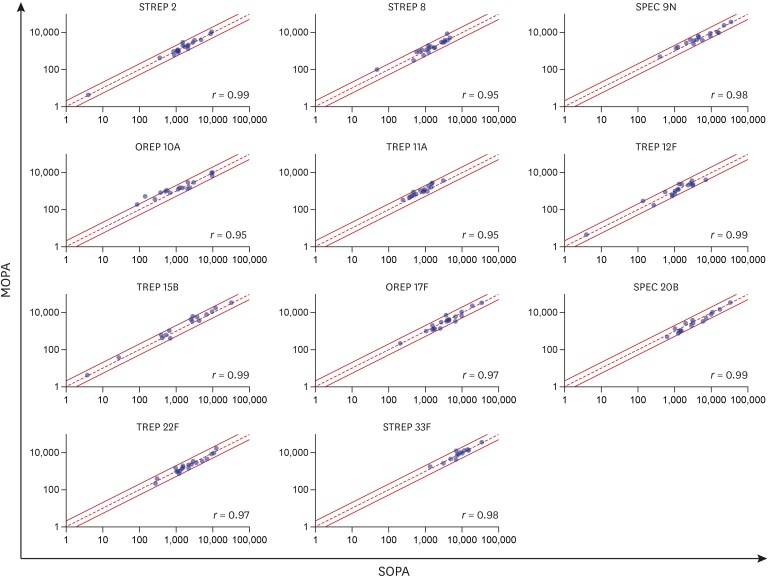
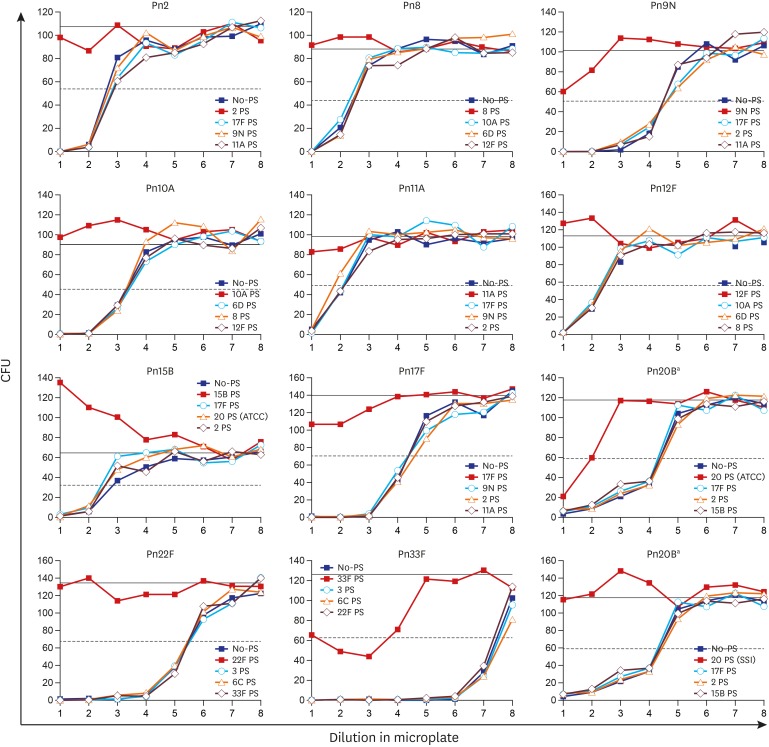
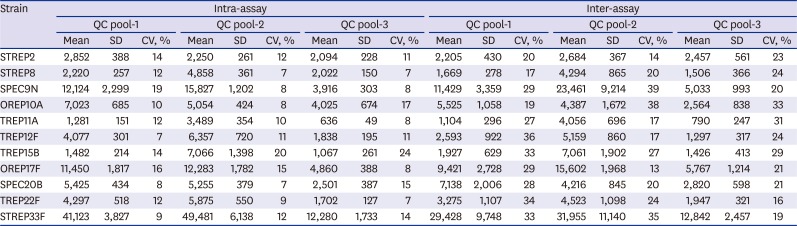
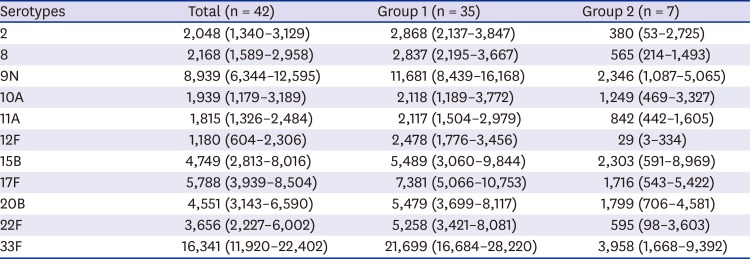




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download