INTRODUCTION
MATERIALS AND METHODS
Participants
Ethics
Date collection
Determination of UGT2B7 -161 genotype
Treatment
Evaluation of cardiotoxicity
Statistics analysis
RESULTS
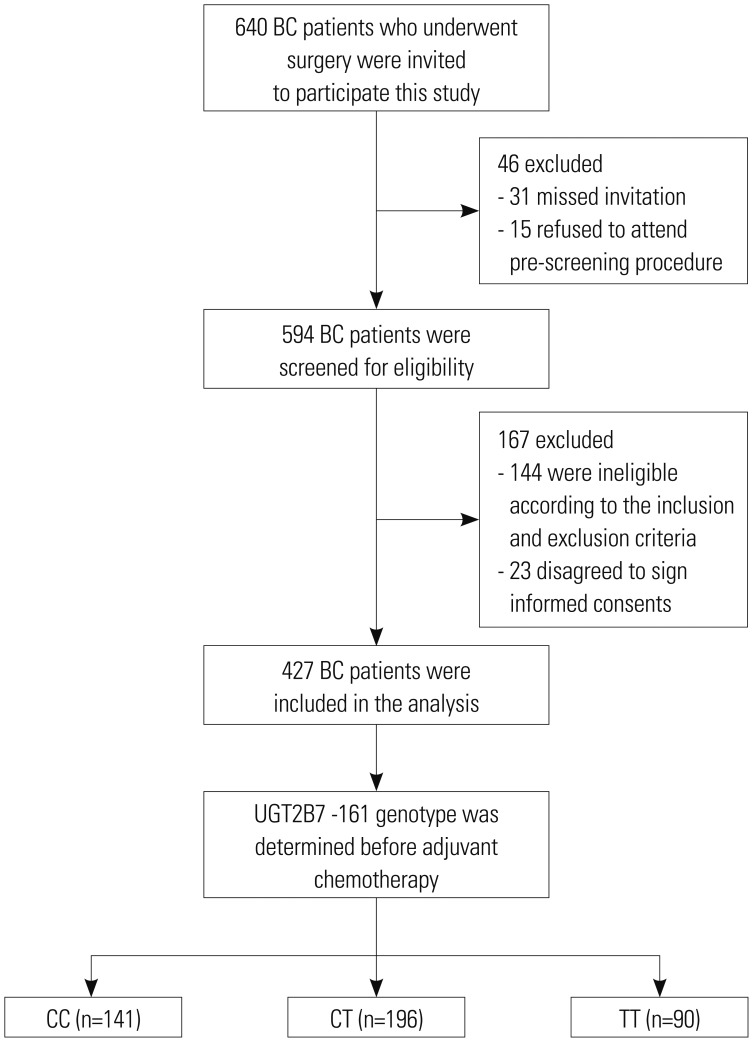
Study flow
Correlation of UGT2B7 -161 C>T genotypes with clinical characteristics
Table 1
Correlation of UGT2B7 -161 Genotype with Clinical Characteristics of Patients
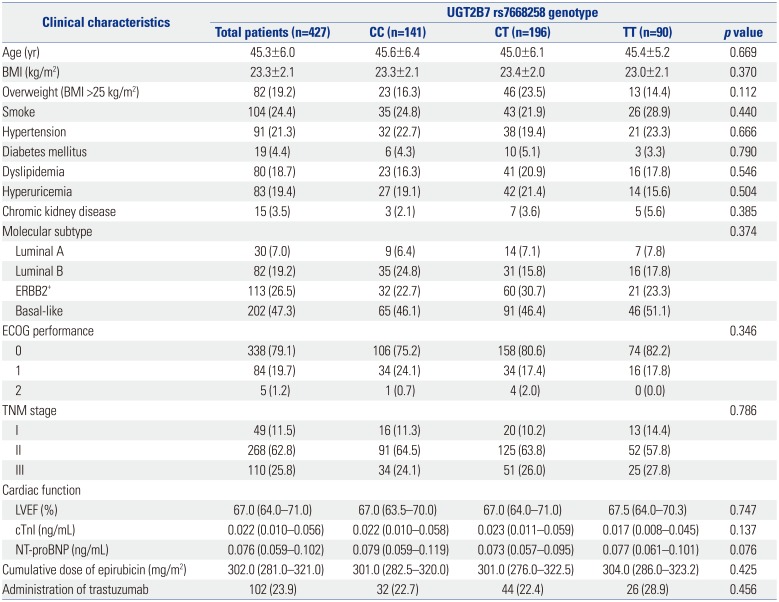
BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; NT-proBNP, N-terminal probrain natriuretic peptide; UGT2B7, uridine glucuronosyltransferase 2B7.
Data are presented as a mean±standard deviation, median (interquartile range), or count (%). Comparison was determined by one-way ANOVA, Kruskal-Wallis H rank sum test, or chi-square test. p value <0.05 was considered significant.
LVEF during and after adjuvant chemotherapy in BC patients
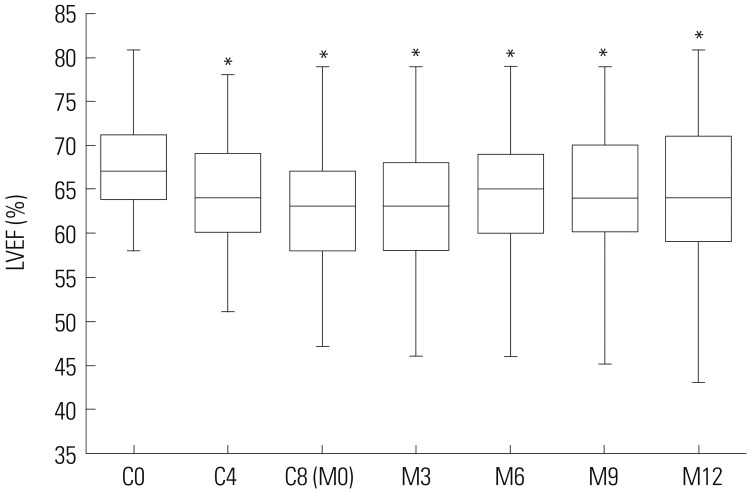 | Fig. 2LVEF during and after adjuvant chemotherapy in BC patients. LVEF values were lower at C4, C8 (M0), M3, M6, M9, and M12, compared to C0 in BC patients undergoing EC-D adjuvant chemotherapy. Comparison between paired time points was performed using Wilcoxon signed-rank sum test. *p<0.001 compared with C0. LVEF, left ventricular ejection fraction; C4/C8, 4th/8th cycle of adjuvant chemotherapy; M3/M6/M9/M12, 3/6/9/12 months after adjuvant chemotherapy; BC, breast cancer. |
Cardiotoxicity after adjuvant chemotherapy in BC patients
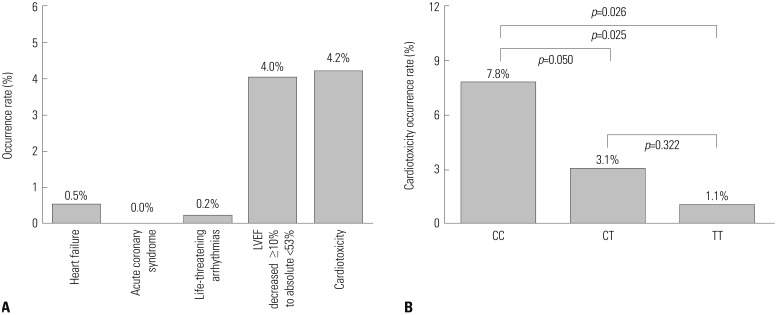 | Fig. 3Occurrence of cardiotoxicity after adjuvant chemotherapy in BC patients. (A) The total occurrence of cardiotoxicity was 4.2% in 427 BC patients, and the percentage of cases presenting with heart failure, acute coronary syndrome, life-threatening arrhythmias, and LVEF decreased ≥10% to absolute <53% were 0.5, 0.0, 0.2, and 4.0%, respectively (Two patients presented with both heart failure and LVEF decreased ≥10% to absolute <53%). (B) The occurrence rate of cardiotoxicity in patients with UGT2B7 -161 TT genotype was 1.1%, which was lower than that in patients with UGT2B7 -161 CT (3.1%) and UGT2B7 -161 CC (7.8%) genotypes. Comparison among three groups was conducted using chi-square test, and p<0.05 was considered significant. BC, breast cancer; LVEF, left ventricular ejection fraction; UGT2B7, uridine glucuronosyltransferase 2B7. |
Factors affecting cardiotoxicity occurrence by univariate logistic regression model analysis in the addictive model
Table 2
Factors Affecting Cardiotoxicity Occurrence in Univariate Logistic Regression Model Analysis (Addictive Model)
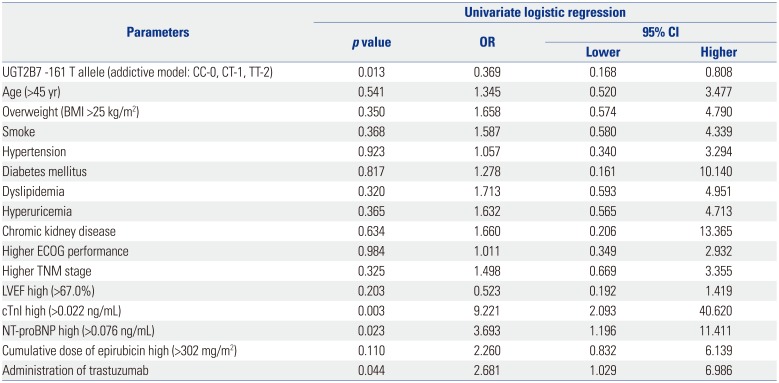
UGT2B7, uridine glucuronosyltransferase 2B7; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio; CI, confidence interval.
Data are presented as a p value or OR and 95% CI. UGT2B7 -161 genotype was analyzed using an addictive model, which was scored as CC-0, CT-1, and TT-2. All continuous variables were stratified by medians respectively. p value <0.05 was considered significant.
Factors affecting cardiotoxicity occurrence by multivariate logistic regression model analysis in the addictive model
Table 3
Factors Affecting Cardiotoxicity Occurrence in Multivariate Logistic Regression Model Analysis (Addictive Model)
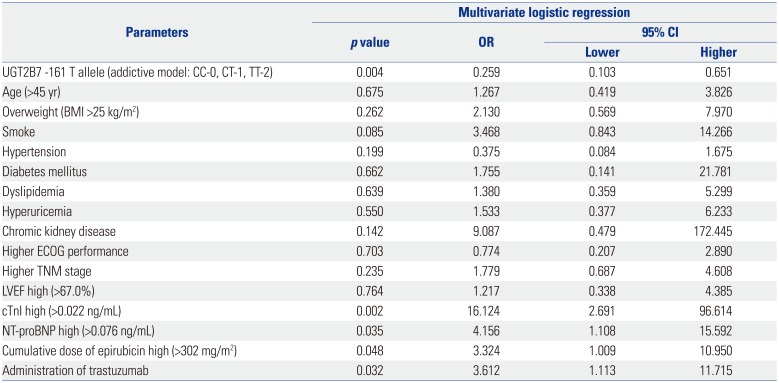
UGT2B7: uridine glucuronosyltransferase 2B7, BMI: body mass index; ECOG, Eastern Cooperative Oncology Group; LVEF, left ventricular ejection fraction; cTnI, cardiac troponin I; NT-proBNP, N-terminal pro-brain natriuretic peptide; OR, odds ratio; CI, confidence interval.
Data are presented as a p value or OR and 95% CI. UGT2B7 -161 genotype was analyzed using an addictive model, which was scored as CC-0, CT-1, or TT-2. All continuous variables were stratified by medians respectively. p value <0.05 was considered significant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download