Abstract
Skin-related toxicity is one of the most important adverse events from multi-target tyrosine kinase inhibitor (MTKI) to treat radioiodine refractory thyroid cancer. As hand foot skin reaction can limit quality of life and therapeutic effectiveness, it is essential to cope with a variety of severity of skin-related toxicity induced by MTKI. Herein, we will discuss two representative cases of skin-related toxicities which were managed by discontinuation/reduction of therapeutic doses of MTKI and were treated by proper medication in thyroid cancer patients with distant metastasis.
Figures and Tables
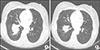 | Fig. 1(A) A 5.0 cm sized large tumor mass with growing nature attached to a bronchus in right lower lobe. (B) After three-month sorafenib treatment, the size of large metastatic mass was reduced to 4.0 cm, without bleeding. |
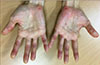 | Fig. 2Three weeks after start of sorafenib 800 mg per day, periungual swelling and multiple painful erythema occurred on both hands, indicating hand foot skin reaction (grade 3). |
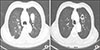 | Fig. 3(A) One 3.5 cm sized growing metastatic pulmonary mass in left lower lobe was refractory to sorafenib. (B) Tumor cavitation with decreased size (2.5 cm) occurred on three months after lenvatinib treatment. |
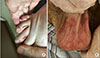 | Fig. 4(A) Erythematous swelling and pruritus with several bleeding creases on right toes occurred on two-month lenvatinib treatment (14 mg per day). (B) Scrotal eczema showing erythematous plaques with pruritus on two-month lenvatinib treatment (14 mg per day). |
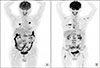 | Fig. 5(A) Dominant two pulmonary metastatic nodules with small multiple spine metastases on PET/CT scan before lenvatinib treatment. (B) Progressive widespread metastatic lesions on whole body, including multiple spine lesions and soft tissue metastasis on PET/CT scan (three months after lenvatinib treatment). |
References
1. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–328.

2. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodinerefractory thyroid cancer. N Engl J Med. 2015; 372(7):621–630.

3. Chanprapaph K, Rutnin S, Vachiramon V. Multikinase inhibitor-induced hand-foot skin reaction: A review of clinical presentation, pathogenesis, and management. Am J Clin Dermatol. 2016; 17(4):387–402.

4. Resteghini C, Cavalieri S, Galbiati D, Granata R, Alfieri S, Bergamini C, et al. Management of tyrosine kinase inhibitors (TKI) side effects in differentiated and medullary thyroid cancer patients. Best Pract Res Clin Endocrinol Metab. 2017; 31(3):349–361.

5. Jean GW, Mani RM, Jaffry A, Khan SA. Toxic effects of sorafenib in patients with differentiated thyroid carcinoma compared with other cancers. JAMA Oncol. 2016; 2(4):529–534.

6. Erber R, Thurnher A, Katsen AD, Groth G, Kerger H, Hammes HP, et al. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004; 18(2):338–340.
7. Jain L, Gardner ER, Figg WD, Chernick MS, Kong HH. Lack of association between excretion of sorafenib in sweat and hand-foot skin reaction. Pharmacotherapy. 2010; 30(1):52–56.

8. Brose MS, Cabanillas ME, Cohen EE, Wirth LJ, Riehl T, Yue H, et al. Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016; 17(9):1272–1282.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download