Abstract
Lenvatinib, an oral multi-kinase inhibitor, is a valuable treatment option for advanced differentiated thyroid carcinoma. However, severe treatment-related adverse events occur up to 30% of the patients receiving lenvatinib, making it a challenge for clinicians to maintain this drug and therefore affecting the outcome of therapy. Blood vessel related events, such as hypertension or proteinuria, are among the most frequent adverse events. We present a case of 65-year-old man with radioactive iodine refractory papillary thyroid carcinoma with cervical lymph node metastasis and tracheal invasion receiving lenvatinib who developed proteinuria and worsening of hypertension. Management with repeated dose reductions and using supportive medications allowed this patient to continue lenvatinib with his disease stably controlled. Early detection of patients at risk for these adverse events and cautious administration of lenvatinib at appropriate level are crucial in managing patients receiving lenvatinib.
Figures and Tables
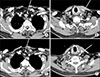 | Fig. 1The radiologic findings of the recurrent tumor in posterior wall of upper thoracic trachea and metastatic cervical lymph nodes. (A, B) Baseline images before initiation of lenvatinib. (C, D) Images of eight months after show decreased extent of the lesions. Arrows pointing for the tracheal lesion (A, C) and cervical lymph node (B, D). |
References
1. Tohyama O, Matsui J, Kodama K, Hata-Sugi N, Kimura T, Okamoto K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014; 2014:638747.

2. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res. 2008; 14(17):5459–5465.

3. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372(7):621–630.

4. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–328.

5. Kiyota N, Schlumberger M, Muro K, Ando Y, Takahashi S, Kawai Y, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015; 106(12):1714–1721.

6. Eremina V, Cui S, Gerber H, Ferrara N, Haigh J, Nagy A, et al. Vascular endothelial growth factor a signaling in the podocyte-endothelial compartment is required for mesangial cell migration and survival. J Am Soc Nephrol. 2006; 17(3):724–735.

7. Bollee G, Patey N, Cazajous G, Robert C, Goujon JM, Fakhouri F, et al. Thrombotic microangiopathy secondary to VEGF pathway inhibition by sunitinib. Nephrol Dial Transplant. 2009; 24(2):682–685.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download