This article has been corrected. See "Corrigendum: Adverse Events of Tyrosine Kinase Inhibitors in Patients with Advanced Thyroid Cancer" in Volume 12 on page 70.
Abstract
Tyrosine kinase inhibitors (TKIs) are widely used for the treatment of advanced radioiodine-refractory thyroid cancer. Although the previous studies including large-scale randomized controlled trials have demonstrated the effects of TKIs in advanced thyroid cancers, it has been found that most patients experienced adverse events (AEs). Unlike other cancers, even patients with advanced thyroid cancers are often asymptomatic. Rather, TKI use can make patients suffer adverse events. Therefore, the use of TKI should be decided after the full consideration of AEs as well as its efficacies. While using TKI, AEs should be monitored, evaluated, and managed appropriately, if AEs develop. In this review, the occurrence, evaluation, and management of AEs of sorafenib, lenvatinib, and vandetanib will be described, which TKIs are most commonly used for the treatment of advanced thyroid cancer. Some suggestions for the management of AEs in the real life are also provided.
Figures and Tables
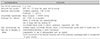
Fig. 1
Adverse event record (Reprint with permission from Reference 20. Copyright 2013 Endocrine Society).

References
1. Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014; 384(9940):319–328.

2. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015; 372(7):621–630.

3. Wells SA Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol. 2012; 30(2):134–141.

4. National Cancer Institute. Protocol development: Cancer Therapy Evaluation Program 2017.
5. Worden F, Fassnacht M, Shi Y, Hadjieva T, Bonichon F, Gao M, et al. Safety and tolerability of sorafenib in patients with radioiodine-refractory thyroid cancer. Endocr Relat Cancer. 2015; 22(6):877–887.

6. Jean GW, Mani RM, Jaffry A, Khan SA. Toxic effects of sorafenib in patients with differentiated thyroid carcinoma compared with other cancers. JAMA Oncol. 2016; 2(4):529–534.

7. Shen CT, Qiu ZL, Luo QY. Sorafenib in the treatment of radioiodine-refractory differentiated thyroid cancer: a meta-analysis. Endocr Relat Cancer. 2014; 21(2):253–261.

8. Kim M, Kim TH, Shin DY, Lim DJ, Kim EY, Kim WB, et al. Tertiary care experience of sorafenib in the treatment of progressive radioiodine-refractory differentiated thyroid carcinoma: a Korean multicenter study. Thyroid. 2018; 28(3):340–348.

9. Haddad RI, Schlumberger M, Wirth LJ, Sherman EJ, Shah MH, Robinson B, et al. Incidence and timing of common adverse events in Lenvatinib-treated patients from the SELECT trial and their association with survival outcomes. Endocrine. 2017; 56(1):121–128.

10. Kiyota N, Schlumberger M, Muro K, Ando Y, Takahashi S, Kawai Y, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci. 2015; 106(12):1714–1721.

11. Brose MS, Worden FP, Newbold KL, Guo M, Hurria A. Effect of age on the efficacy and safety of lenvatinib in radioiodine-refractory differentiated thyroid cancer in the Phase III SELECT trial. J Clin Oncol. 2017; 35(23):2692–2699.

12. Cabanillas ME, Schlumberger M, Jarzab B, Martins RG, Pacini F, Robinson B, et al. A phase 2 trial of lenvatinib (E7080) in advanced, progressive, radioiodine-refractory, differentiated thyroid cancer: A clinical outcomes and biomarker assessment. Cancer. 2015; 121(16):2749–2756.

13. Jasim S, Iniguez-Ariza NM, Hilger CR, Chintakuntlawar AV, Ryder MM, Morris JC 3rd, et al. Optimizing lenvatinib therapy in patients with metastatic radioactive iodine-resistant differentiated thyroid cancers. Endocr Pract. 2017; 23(10):1254–1261.

14. Berdelou A, Borget I, Godbert Y, Nguyen T, Garcia ME, Chougnet CN, et al. Lenvatinib for the treatment of radioiodine-refractory thyroid cancer in real-life practice. Thyroid. 2017; [Epub ahead of print].

15. Bastholt L, Kreissl MC, Fuhrer D, Maia AL, Locati LD, Maciel L, et al. Effect of an outreach programme on vandetanib safety in medullary thyroid cancer. Eur Thyroid J. 2016; 5(3):187–194.

16. Chougnet CN, Borget I, Leboulleux S, de la Fouchardiere C, Bonichon F, Criniere L, et al. Vandetanib for the treatment of advanced medullary thyroid cancer outside a clinical trial: results from a French cohort. Thyroid. 2015; 25(4):386–391.

17. Wells SA Jr, Gosnell JE, Gagel RF, Moley J, Pfister D, Sosa JA, et al. Vandetanib for the treatment of patients with locally advanced or metastatic hereditary medullary thyroid cancer. J Clin Oncol. 2010; 28(5):767–772.

18. Massicotte MH, Borget I, Broutin S, Baracos VE, Leboulleux S, Baudin E, et al. Body composition variation and impact of low skeletal muscle mass in patients with advanced medullary thyroid carcinoma treated with vandetanib: results from a placebo-controlled study. J Clin Endocrinol Metab. 2013; 98(6):2401–2408.

19. Giacchero D, Ramacciotti C, Arnault JP, Brassard M, Baudin E, Maksimovic L, et al. A new spectrum of skin toxic effects associated with the multikinase inhibitor vandetanib. Arch Dermatol. 2012; 148(12):1418–1420.

20. Carhill AA, Cabanillas ME, Jimenez C, Waguespack SG, Habra MA, Hu M, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab. 2013; 98(1):31–42.

21. Ghatalia P, Je Y, Kaymakcalan MD, Sonpavde G, Choueiri TK. QTc interval prolongation with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Br J Cancer. 2015; 112(2):296–305.

22. Shah RR, Morganroth J, Shah DR. Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf. 2013; 36(5):295–316.

23. Dadu R, Waguespack SG, Sherman SI, Hu MI, Busaidy NL, Jimenez C, et al. Efficacy and tolerability of different starting doses of sorafenib in patients with differentiated thyroid cancer. Oncologist. 2014; 19(5):477–482.

24. ClinicalTrials.gov. A phase 2 trial of lenvatinib (E7080) in subjects with iodine-131 refractory differentiated thyroid cancer to evaluate whether an oral starting dose of 18 mg daily will provide comparable efficacy to a 24 mg starting dose, but have a better safety profile. cited November 17, 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT02702388.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download