초록
Background and objectives
Salivary hypofunction is one of the common side effects after radioiodine therapy, and its pathophysiology is salivary ductal stenosis resulting from ductal cell injury. This study aimed to develop the functional culture environment of human parotid gland ductal cells in in vitro three-dimensional perfusion culture system.
Materials and Methods
We compared plastic dish culture method and three-dimensional culture system containing Matrigel and nanofiber. Morphogenesis of reconstituted salivary structures was assessed by histomorphometry. Functional characteristics were assessed by immunohistochemistry and reverse transcription polymerase chain reaction (aquaporin 5, CK7, CK18, connexin 43, and p21). In addition, we designed the media perfusion culture system and identified higher rate of cell proliferation and expression of connexin 43 in perfusion system comparing to dish.
Results
Human parotid ductal cells were well proliferated with the ductal cell characters under environment with Matrigel. In the presence of Matrigel, aquaporin 5, CK18 and connexin 43 were more expressed than 2D dish and 3D nanofiber setting. In the media perfusion culture system, ductal cells in 3D culture media showed higher cells count and connexin 43 expression compared to 2D dish.
Go to : 
REFERENCES
1). Grewal RK, Larson SM, Pentlow CE, Pentlow KS, Gonen M, Qualey R, et al. Salivary gland side effects commonly develop several weeks after initial radioactive iodine ablation. J Nucl Med. 2009; 50(10):1605–10.

2). Choi JS, Park IS, Kim SK, Lim JY, Kim YM. Morphometric and functional changes of salivary gland dysfunction after radioactive iodine ablation in a murine model. Thyroid. 2013; 23(11):1445–51.

3). La Perle KM, Kim DC, Hall NC, Bobbey A, Shen DH, Nagy RS, et al. Modulation of sodium/iodide symporter expression in the salivary gland. Thyroid. 2013; 23(8):1029–36.

4). Joraku A, Sullivan CA, Yoo J, Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007; 75(4):318–24.

5). Nielsen MS, Axelsen LN, Sorgen PL, Verma V, Delmar M, Holstein-Rathlou NH. Gap junctions. Compr Physiol. 2012; 2(3):1981–2035.

6). Kuraoka A, Yamanaka I, Miyahara A, Shibata Y, Uemura T. Immunocytochemical studies of major gap junction proteins in rat salivary glands. Eur Arch Otorhinolaryngol. 1994; 251(Suppl 1):S95–9.

7). Muramatsu T, Hashimoto S, Shimono M. Differential expression of gap junction proteins connexin32 and 43 in rat submandibular and sublingual glands. J Histochem Cytochem. 1996; 44(1):49–56.

8). Shimono M, Young Lee C, Matsuzaki H, Ishikawa H, Inoue T, Hashimoto S, et al. Connexins in salivary glands. Eur J Morphol. 2000; 38(4):257–61.

9). Chan YH, Huang TW, Young TH, Lou PJ. Selective culture of different types of human parotid gland cells. Head Neck. 2011; 33(3):407–14.

10). Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009; 92(3):466–71.

11). Hardman P, Spooner BS. Localization of extracellular matrix components in developing mouse salivary glands by confocal microscopy. Anat Rec. 1992; 234(3):452–9.

12). Wang S, Cukierman E, Swaim WD, Yamada KM, Baum BJ. Extracellular matrix protein-induced changes in human salivary epithelial cell organization and proliferation on a model biological substratum. Biomaterials. 1999; 20(11):1043–9.

13). Bing SJ, Kim MJ, Ahn G, Im J, Kim DS, Ha D, et al. Acidic polysaccharide of Panax ginseng regulates the mitochondria/caspase- dependent apoptotic pathway in radiation-induced damage to the jejunum in mice. Acta Histochem. 2014; 116(3):514–21.
14). Tran SD, Wang J, Bandyopadhyay BC, Redman RS, Dutra A, Pak E, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005; 11(1-2):172–81.

15). Szlavik V, Szabo B, Vicsek T, Barabas J, Bogdan S, Gresz V, et al. Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Eng Part A. 2008; 14(11):1915–26.
Go to : 
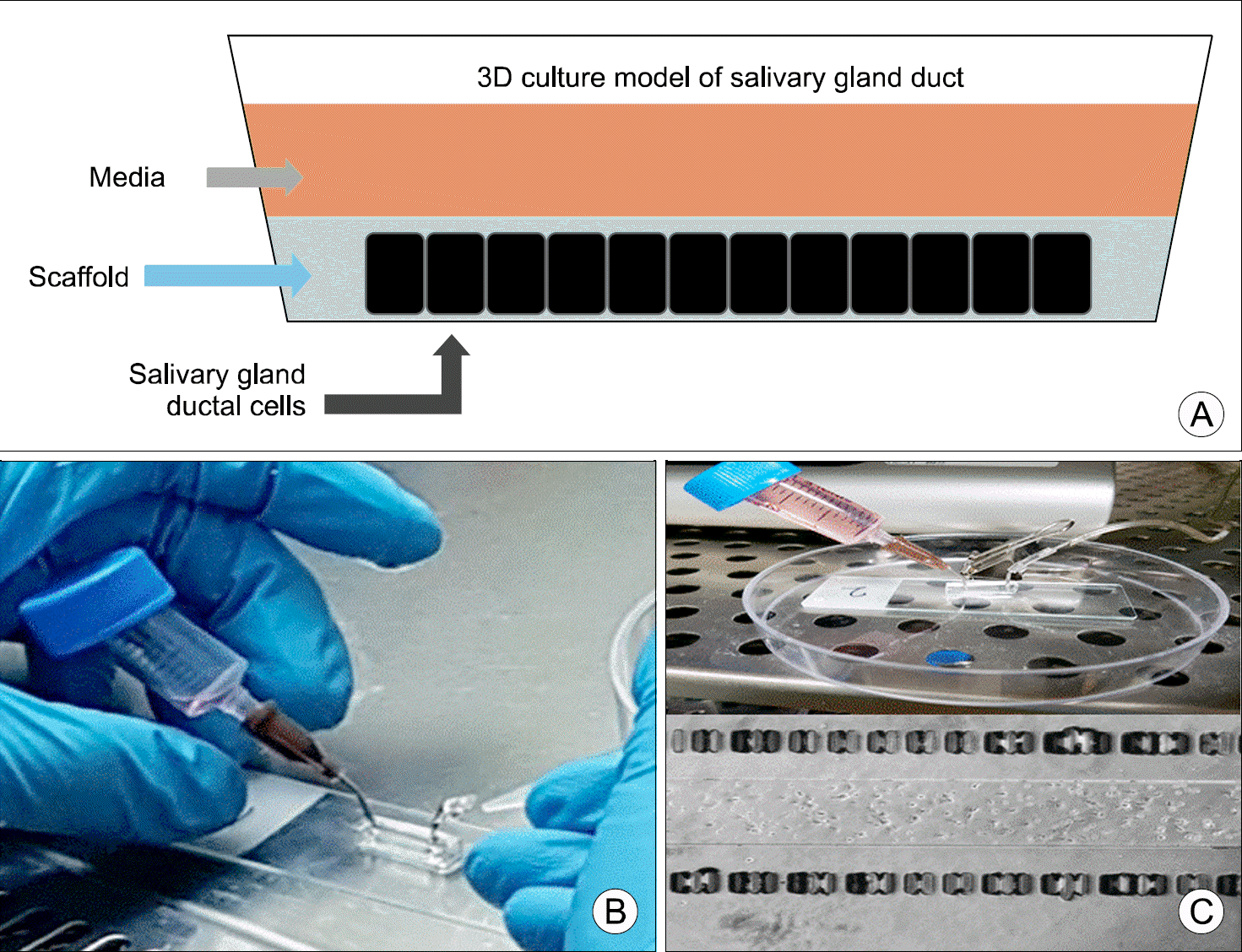 | Fig. 1.A schematic diagram of a three-dimensional culture model of ductal cells (A), photographs of ductal cell perfusion devices (B), and optical microscopic photograph of ductal cell culture (C). |
 | Fig. 2.(A) The morphology of salivary ductal cells according to 2-D and 3-D culture conditions, (B) The mRNA expression of acinar cell marker (AQP5), ductal cell markers (CK7,18) and cell junctional protein (connexin 43) (∗p<0.05, ∗∗ p<0.01, ∗∗∗p<0.001). |
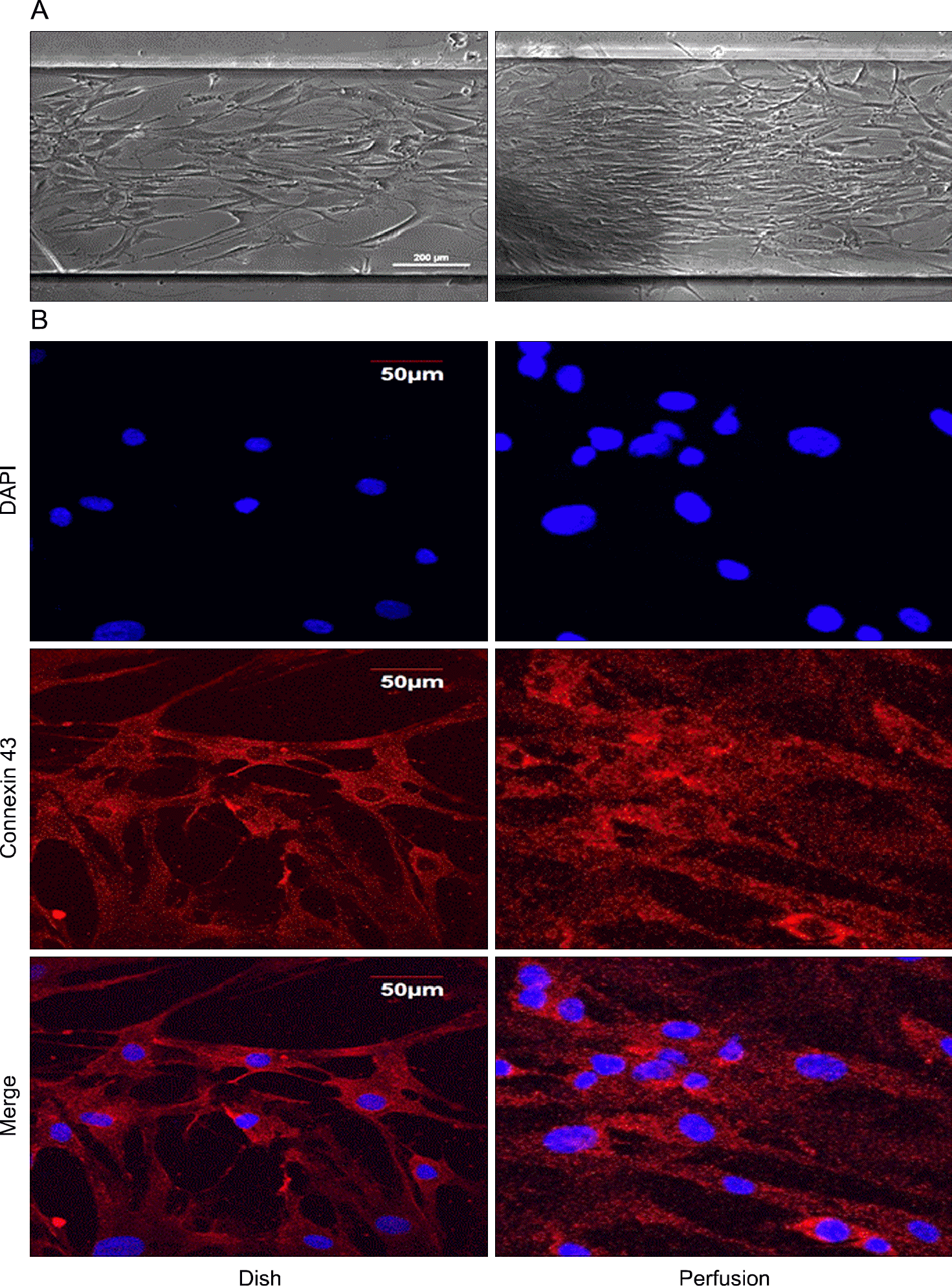 | Fig. 3.Conformational change of salivary ductal cells under perfusion culture system of ductal cells (A), confirmation of cell gap junctional protein (connexin 43) expression in two-dimensional culture and perfusion culture (B). |
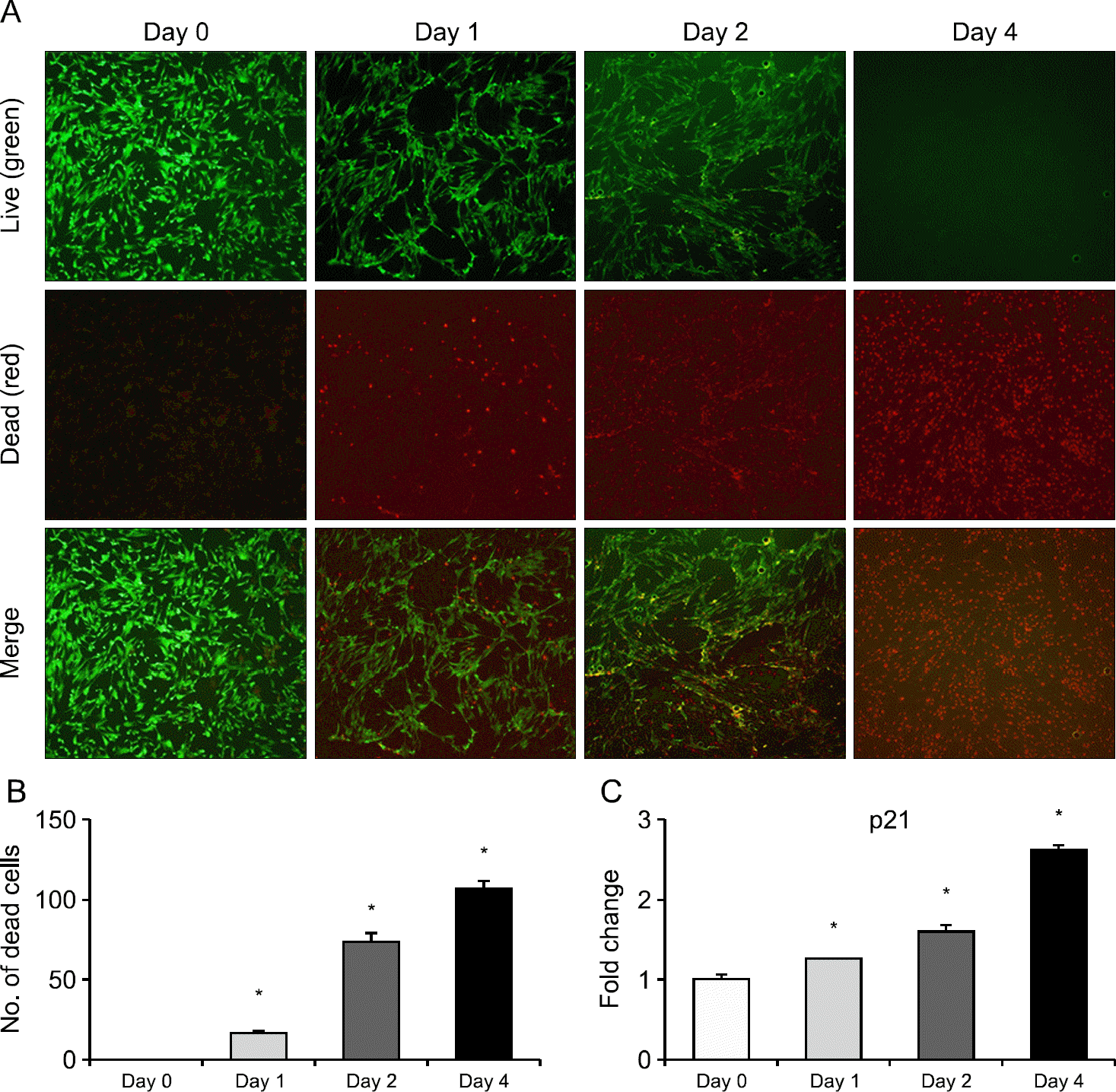 | Fig. 4.(A, B) Viability of salivary duct cells over time after radioiodine treatment. (C) Expression of p21 over time after radioiodine treatment (∗p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download