Abstract
Background and Objectives
Sodium-iodine symporter (NIS) is a marker for the degree of differentiation in thyroid cancer. The genetic factors or microenvironment surrounding tumors can affect transcription of NIS. In this study, we investigated the NIS mRNA expression according to mutational status and coexistent lymphocytic thyroiditis in papillary thyroid cancer (PTC).
Materials and Methods
The RNA expression levels of NIS in the samples from database of The Caner Genome Atlas (TCGA; n=494) and our institute (n=125) were analyzed.
Results
The PTCs with the BRAFV600E mutation and the coexistence of BRAFV600E and TERT promoter mutations showed significantly lower expression of NIS (p<0.001, respectively), and those with BRAF-like molecular subtype also had reduced expression of NIS (p<0.001). NIS expression showed a positive correlation with thyroid differentiation score (r=0.593, p<0.001) and negative correlations with expressions of genes involved in ERK signaling (r=−0.164, p<0.001) and GLUT-1 gene (r=−0.204, p<0.001). The PTCs with lymphocytic thyroiditis showed significantly higher NIS expression (p=0.013), regardless of mutational status.
The sodium-iodide symporter (NIS) is a transmembrane glycoprotein that mediates active transport of iodide into thyroid follicular cells and cancer cells, the key mechanism of radioactive iodine (RAI) therapy in thyroid cancer. Dedifferentiation of thyroid cancer is related to the molecular alterations of proteins involved in iodine metabolism, including NIS, which results in the RAI-refractoriness.1) The NIS transcription can be modulated by genetic or epigenetic factors.2) The comprehensive studies of the genomic landscape of thyroid cancer reported that there were the distinct signaling and transcriptomic consequences according to the molecular subtypes, and BRAFV600E-like molecular subtype tumors showed lower expression of iodine metabolism genes and higher transcriptional output of mitogen-activated protein kinase/extracellular-signal-regulated kinase (MAPK/ERK) signaling compared to the tumors of RAS-like and non-BRAF-non-RAS subtypes.34) This suggests that the mutational status may affect NIS expression, which can be a key clue to determine the therapeutic effect of RAI. In this study, we focused on SLC5A5 (NIS mRNA) expression in papillary thyroid carcinoma (PTC) according to the mutational status or coexistent lymphocytic thyroiditis (LT), which reflects changes in tumor microenvironment.
The data on somatic mutations, mRNA expression, and clinical information of 494 patients with PTC of The Cancer Genome Atlas (TCGA) study were downloaded from the UCSC Cancer Browser (https://genome-cancer.ucsc.edu). The mRNA expression data, which were generated using the Illumina HiSeq V2 platform, were presented as reads per kilobase million (RPKM). A total of 387 samples had the TERT promoter sequencing results from either Illumina MiSeq or whole genome sequencing. For validation, 125 patients with PTC (77 classical PTC and 48 follicular variant of PTC) whose RNA sequencing data was available from our previous research4) (hereafter, Seoul National University Hospital [SNUH] database) were included. The RNA sequencing libraries were sequenced on the Illumina HiSeq 2000 platform, and expression values were presented as fragments per kilobase million (FPKM). For detection of TERT promoter mutations, we performed Sanger sequencing by a previously described method.5) This study was approved by the institutional review board of the Seoul National University Hospital, and was conducted in accordance with the Declaration of Helsinki (approved ID: H-1108-041-372).
We defined ‘BRAF-like’ and ‘RAS-like’ molecular subtypes, as was done in the previous studies,34) in which the tumor gene expression profile resembles either the BRAF or RAS mutant profile.
Thyroid differentiation score (TDS) and ERK signature were also calculated by methods in the previous studies.34) The TDS ranks the PTC samples according to mRNA expression levels of a set of 16 thyroid function genes (iodine metabolism genes), and reflects the degree of differentiation of samples. The ERK signature is derived from mRNA expression levels of a set of 52 MAPK signaling pathway genes, and represents activation level of MAPK/ERK signaling pathway.
To compare the mRNA expression levels of NIS among groups, the Wilcoxon-Mann-Whitney test or the Kruskal-Wallis test was used. For comparisons of TDS, ERK signature and SLC2A1 (GLUT-1 mRNA) expression levels according to mutational status, the analysis of variance test was used. The post hoc test was performed by the Bonferroni method. Relationship between NIS expression and TDS, ERK signature, or GLUT-1 expression were evaluated by Pearson's correlation coefficient. Statistical significance was defined as two-sided p values <0.05. All statistical analyses were performed in R v3.4.2 (www.r-project.org).
We compared NIS mRNA expression levels according to the well-known driver mutations, which are associated with prognosis of thyroid cancer, BRAFV600E, RAS, and TERT promoter mutations. Among 494 PTC samples from TCGA study, the BRAFV600E and RAS mutations were found in 234 (47.4%) and 52 (10.2%) PTCs, respectively. TERT promoter mutations were observed in 39 of 387 (10.1%) PTCs. The PTCs with the BRAFV600E mutation showed significantly lower expression of NIS (p<0.001, Fig. 1A), while those with RAS mutations showed relatively higher NIS expression, although not statistically significant (p=0.607, Fig. 1B). In the case of TERT promoter mutations, expression of NIS was significantly decreased (p<0.001, Fig. 1C), similarly to the BRAFV600E mutation. When analyzed by considering the status of all three mutations, NIS expression was decreased in the groups of BRAF only (p<0.001) and BRAF+TERT (p<0.001) in comparison with the no mutation group (Fig. 1D). The TERT only and RAS+TERT groups showed lower NIS expression levels, even though there was no statistically significant difference due to the small number of samples in the groups. When the PTC samples were classified as BRAF-like or RAS-like subtype according to the gene expression pattern, we also confirmed that NIS expression was reduced in samples with BRAF-like molecular subtype (p<0.001, Fig. 1E).
Among 125 PTC samples from the SNUH database, 67 (53.6%) and 24 (19.2%) PTCs had the BRAFV600E and RAS mutations, respectively. TERT promoter mutations were found in 12 of 96 (12.5%) PTCs. The PTCs with BRAFV600E or RAS mutations had a tendency of lower NIS expression compared to the wild-type PTCs or those with TERT promoter mutations, but there was no statistically significance (Fig. 1F–I). NIS expression did not differ between BRAF-like and RAS-like molecular subtypes (Fig. 1J).
Next, to identify the associations of NIS mRNA expression with thyroid differentiation and MAPK/ERK signaling pathway which is activated by the BRAFV600E mutation, we used the gene set enrichment scores such as TDS and ERK signature. Moreover, we examined the relationship between NIS and GLUT-1 mRNA expressions, because the previous studies678) reported that GLUT-1 expression is elevated in less differentiated thyroid cancer, in contrast to NIS. In TCGA database, NIS mRNA expression was positively correlated with TDS which represents expression of iodine metabolism genes (r=0.593, p<0.001; Fig. 2A), and negatively correlated with ERK signature (r=−0.164, p<0.001; Fig. 2B) and GLUT-1 mRNA expression (r=−0.204, p<0.001; Fig. 2C). In the SNUH database, NIS mRNA expression showed a positive correlation with TDS (r=0.242, p=0.007) and a negative correlation with ERK signature (r=−0.381, p<0.001), however, there was no significant association with GLUT-1 mRNA expression (r=−0.066, p=0.464).
As previously reported, BRAFV600E or TERT promoter mutations occurred less-differentiated PTCs, that is, TDS was lower in the groups with BRAF only (p<0.001), TERT only (p=0.001), or BRAF+TERT (p<0.001) compared to the no mutation group (Fig. 2D). The pattern of NIS mRNA expression was similar to that of TDS (Fig. 2D). In contrast, ERK signature and GLUT-1 mRNA expression were higher in the BRAF only and BRAF+TERT groups compared to the no mutation group (p<0.001, respectively; Fig. 2D).
In a previous study of 134 PTC samples, immunohistochemistry was performed to detect NIS expression, and the BRAFV600E mutation was significantly associated with a lower expression of NIS in PTCs without Hashimoto's thyroiditis, but not in those with Hashimoto's thyroiditis.9) Therefore, we investigated the association between NIS mRNA expression and LT. Interestingly, in TCGA dataset, PTC samples with LT showed significantly higher NIS expression (p=0.013, Fig. 3A). Furthermore, regardless of mutational status of BRAFV600E, RAS, and TERT, NIS expression was relatively preserved in LT-positive samples (Fig. 3B–D). When we analyzed the expression according to BRAF-like and RAS-like molecular subtypes combined with LT status, there was a gradually increasing trend: BRAF-like without LT < BRAF-like with LT < RAS-like without LT < RAS-like with LT (p for trend <0.001, Fig. 3E).
In PTCs with the BRAFV600E mutation, NIS mRNA expression was reduced, suggesting that BRAFV600E might be involved in the dedifferentiation of tumors. The BRAFV600E activated the MAPK/ERK pathway and decreased the expression of iodine metabolism-related genes including NIS, and the GLUT-1 gene expression was increased with decreasing NIS expression. On the other hand, in PTC with LT, NIS mRNA expression was increased, indicating that LT might play a role of restoring NIS expression.
The BRAFV600E mutation is associated with a lower NIS expression and lower RAI uptake.1011) We confirmed this using a large number of RNA sequencing data of PTC samples. These findings provide an evidence of the use of inhibitors of BRAF-MAPK pathway such as Selumetinib (MEK1/2 inhibitor),12) Dabrafenib or Vemurafenib (BRAF inhibitors)13) for re-differentiation therapy of RAI-refractory thyroid cancer. The mechanisms of RAI-refractory disease include the decreased transcription of NIS by genetic and epigenetic regulation, and the diminished translocation of NIS to the membrane of thyroid cancer cells for functional activity.2) A previous study demonstrated that the NIS protein was retained in the cytoplasm regardless of the BRAFV600E status in the PTC, while it was located at the plasma membrane in normal thyroid tissues. However, levels of NIS transcript were significantly reduced in the BRAFV600E-mutant PTCs than wild-type PTCs.7) Regarding RAS mutations, the expression of NIS according to RAS mutations tended to increase in TCGA dataset while it seemed to decrease in the SNUH dataset, although both were not statistically significant. However, as shown in Fig. 1D, when we excluded the BRAF- or TERT-mutant samples from the RAS wild-type group to exclude the effect of other driver mutations, the NIS expression of the RAS-mutant samples also tended to be lower than that of wild-type samples in TCGA dataset similar to SNUH dataset, but it was also not statistically significant.
Several studies showed that patients with tumors of positive fluorodeoxyglucose (FDG)-positron emission tomography (PET) scan are less likely to respond to RAI therapy and have a worse prognosis than those with tumors of negative FDG-PET scan.1415) This called ‘flip-flop phenomenon’, uptake of iodine-131 with no FDG uptake and vice versa.16) In this study, there was a significant negative correlation between NIS and GLUT-1 expressions, which can explain this phenomenon. The BRAFV600E mutation may lead to less-differentiated phenotype and lower expression of iodine metabolism genes, while enhancing glucose uptake and metabolism.
The effects of LT on PTC and its prognosis have been controversial. However, in the majority of related studies,17181920) PTCs with coexisting LT was associated with a lower frequency of extrathyroidal extension and lymph node metastasis, lower pathologic stages, and favorable prognosis, which was demonstrated in a meta-analysis.21) Moreover, other studies showed that the presence of LT was a protective factor against aggressiveness and progression of PTC, even in BRAF-mutant patients.2223) The precise mechanism of this protective effect of LT has not been described yet, but one of the possible mechanisms is that infiltrated lymphocytes may cause tumor suppressive immune reactions such as directly killing cancer cells or inhibiting thyroid cancer cell growth by secreting cytokines. We showed that LT was associated with restoration of NIS expression regardless of mutational status. Our result may be another mechanism to explain the effects of LT on the favorable clinical outcomes of PTC. Additional study is needed to validate the mechanism.
In conclusion, the NIS expression was reduced by the BRAFV600E mutation and a subsequent MAPK/ERK pathway activation, but restored by the presence of LT. This may support the possible mechanism of the more favorable prognosis of PTC patients with LT regardless of BRAFV600E mutation status.
Figures and Tables
 | Fig. 1NIS mRNA expression according to mutational status. (A–E) The mRNA expression levels of SLC5A5 (NIS) from The Cancer Genome Atlas (TCGA) database. Median expression levels of NIS according to mutational status of BRAFV600E (A; wild-type, n=260; mutant-type, n=234), RAS (B; wild-type, n=442; mutant-type, n=52), TERT promoter (C; wild-type, n=348; mutant-type, n=39), their combination (D; none, n=107; BRAF, n=198; RAS, n=43; TERT, n=5; BRAF+TERT, n=28; RAS+TERT, n=6), and molecular subtype (E; BRAF-like, n=272; RAS-like, n=119). (F–J) The mRNA expression levels of NIS from the Seoul National University Hospital (SNUH) database. Median expression levels of NIS according to mutational status of BRAFV600E (F; wild-type, n=58; mutant-type, n=67), RAS (G; wild-type, n=101; mutant-type, n=24), TERT promoter (H; wild-type, n=84; mutant-type, n=12), their combination (I; none, n=15; BRAF, n=50; RAS, n=19; TERT, n=3; BRAF+TERT, n=7; RAS+TERT, n=2), and molecular subtype (J; BRAF-like, n=81; RAS-like, n=35). SLC5A5: NIS gene, WT: wild-type, MUT: mutant-type, B+T: BRAF+TERT, R+T: RAS+TERT, RPKM: reads per kilobase million, FPKM: fragments per kilobase million |
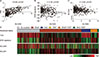 | Fig. 2Associations of NIS mRNA expression with thyroid differentiation score, ERK signature, and GLUT-1 mRNA expression. (A–C) The correlations between SLC5A5 (NIS) mRNA expression and thyroid differentiation score (TDS) (A), ERK signature (B), SLC2A1 (GLUT-1) mRNA expression (C) from TCGA database. The mRNA expression levels were presented as reads per kilobase million (RPKM). r=the Pearson correlation coefficient. (D) The patterns of NIS expression, TDS, ERK signature, and GLUT-1 expression according to mutational status from TCGA database. SLC5A5: NIS gene, TDS: thyroid differentiation score, SLC2A1: GLUT-1 gene |
 | Fig. 3Effects of lymphocytic thyroiditis on NIS mRNA expression. (A–E) The mRNA expression levels of SLC5A5 (NIS) from TCGA database. Median expression levels of NIS according to the status of LT (A; LT(−), n=233; LT(+), n=114), BRAFV600E and LT (B; B(−)LT(−), n=109; B(−)LT(+), n=47; B(+)LT(−), n=124; B(+)LT(+), n=67), RAS and LT (C; R(−)LT(−), n=211; R(−)LT(+), n=113; R(+)LT(−), n=22; R(+)LT(+), n=1), TERT and LT (D; T(−)LT(−), n=157; T(−)LT(+), n=85; T(+)LT(−), n=20; T(+)LT(+), n=6), and molecular subtype and LT (E; BL/LT(−), n=142; BL/LT(+), n=80; RL/LT(−), n=38; RL/LT(+), n=87). (F–J) The mRNA expression levels of NIS from the SNUH database. Median expression levels of NIS according to the status of LT (F; LT(−), n=72; LT(+), n=53), BRAFV600E and LT (G; B(−)LT(−), n=36; B(−)LT(+), n=22; B(+)LT(−), n=36; B(+)LT(+), n=31), RAS and LT (H; R(−)LT(−), n=54; R(−)LT(+), n=47; R(+)LT(−), n=18; R(+)LT(+), n=6), TERT and LT (I; T(−)LT(−), n=51; T(−)LT(+), n=33; T(+)LT(−), n=8; T(+)LT(+), n=4), and molecular subtype and LT (J; BL/LT(−), n=40; BL/LT(+), n=41; RL/LT(−), n=28; RL/LT(+), n=7). SLC5A5: NIS gene, LT: lymphocytic thyroiditis, B: BRAFV600E mutation, R: RAS mutations, T: TERT promoter mutations, BL: BRAF-like, RL: RAS-like, RPKM: reads per kilobase million, FPKM: fragments per kilobase million |
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (grant number: NRF-2016R1A2B4012417).
References
1. Schlumberger M, Lacroix L, Russo D, Filetti S, Bidart JM. Defects in iodide metabolism in thyroid cancer and implications for the follow-up and treatment of patients. Nat Clin Pract Endocrinol Metab. 2007; 3(3):260–269.

2. Spitzweg C, Bible KC, Hofbauer LC, Morris JC. Advanced radioiodine-refractory differentiated thyroid cancer: the sodium iodide symporter and other emerging therapeutic targets. Lancet Diabetes Endocrinol. 2014; 2(10):830–842.

3. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159(3):676–690.
4. Yoo SK, Lee S, Kim SJ, Jee HG, Kim BA, Cho H, et al. Comprehensive analysis of the transcriptional and mutational landscape of follicular and papillary thyroid cancers. PLoS Genet. 2016; 12(8):e1006239.

5. Song YS, Lim JA, Choi H, Won JK, Moon JH, Cho SW, et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer. 2016; 122(9):1370–1379.

6. Lazar V, Bidart JM, Caillou B, Mahe C, Lacroix L, Filetti S, et al. Expression of the Na+/I− symporter gene in human thyroid tumors: a comparison study with other thyroid-specific genes. J Clin Endocrinol Metab. 1999; 84(9):3228–3234.

7. Durante C, Puxeddu E, Ferretti E, Morisi R, Moretti S, Bruno R, et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007; 92(7):2840–2843.

8. Kim S, Chung JK, Min HS, Kang JH, Park DJ, Jeong JM, et al. Expression patterns of glucose transporter-1 gene and thyroid specific genes in human papillary thyroid carcinoma. Nucl Med Mol Imaging. 2014; 48(2):91–97.

9. Dong H, Shen WZ, Yan YJ, Yi JL, Zhang L. Effects of BRAF(V600E) mutation on Na(+)/I(−) symporter expression in papillary thyroid carcinoma. J Huazhong Univ Sci Technolog Med Sci. 2016; 36(1):77–81.

10. Chakravarty D, Santos E, Ryder M, Knauf JA, Liao XH, West BL, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011; 121(12):4700–4711.

11. Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009; 69(11):4885–4893.

12. Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013; 368(7):623–632.

13. Rothenberg SM, Daniels GH, Wirth LJ. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib-response. Clin Cancer Res. 2015; 21(24):5640–5641.

14. Wang W, Larson SM, Tuttle RM, Kalaigian H, Kolbert K, Sonenberg M, et al. Resistance of [18f]-fluorodeoxyglucose-avid metastatic thyroid cancer lesions to treatment with high-dose radioactive iodine. Thyroid. 2001; 11(12):1169–1175.

15. Robbins RJ, Wan Q, Grewal RK, Reibke R, Gonen M, Strauss HW, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006; 91(2):498–505.

16. Feine U, Lietzenmayer R, Hanke JP, Held J, Wohrle H, Muller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996; 37(9):1468–1472.
17. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999; 84(2):458–463.

18. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009; 71(4):581–586.

19. Lang BH, Chai YJ, Cowling BJ, Min HS, Lee KE, Youn YK. Is BRAFV600E mutation a marker for central nodal metastasis in small papillary thyroid carcinoma? Endocr Relat Cancer. 2014; 21(2):285–295.

20. Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, et al. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012; 27(8):883–889.

21. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013; 168(3):343–349.

22. Marotta V, Guerra A, Zatelli MC, Uberti ED, Di Stasi V, Faggiano A, et al. BRAF mutation positive papillary thyroid carcinoma is less advanced when Hashimoto's thyroiditis lymphocytic infiltration is present. Clin Endocrinol (Oxf). 2013; 79(5):733–738.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download