초록
Background and Objectives
Sirtuins (SIRTs) play important roles in cellular and organismal homeostasis. They have distinct gene expression patterns in various cancers; however, the relationship between SIRT expression and the progression of thyroid cancer is unclear. We investigated the expression of SIRTs in patients with papillary thyroid carcinoma (PTC) and their role as biomarkers for predicting the aggressiveness of this disease.
Materials and Methods
We used immunohistochemical staining to evaluate the expression of SIRT1 and SIRT3 in tumor specimens from 270 patients with PTC. We also evaluated the potential association between SIRT expression and diverse clinicopathological features.
Results
High SIRT1 expression was negatively correlated with lymphovascular invasion, central lymph node metastasis, and lateral lymph node metastasis. Multivariate analyses revealed that high SIRT1 expression was a negative independent risk factor for lateral lymph node metastasis. By contrast, high SIRT3 expression was positively correlated with locoregional recurrence. Interes-tingly, when patients were grouped by tumor SIRT expression patterns, the group with low SIRT1 expression and high SIRT3 expression was correlated with more aggressive cancer phenotypes including central lymph node metastasis and lateral lymph node metastasis.
Go to : 
REFERENCES
1). Guarente L. Franklin H. Epstein lecture: sirtuins, aging, and medicine. N Engl J Med. 2011; 364(23):2235–44.
2). Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 2000; 273(2):793–8.

3). Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012; 21(3):297–308.

4). Chalkiadaki A, Guarente L. The multifaceted functions of sirtuins in cancer. Nat Rev Cancer. 2015; 15(10):608–24.

6). Mirebeau-Prunier D, Le Pennec S, Jacques C, Fontaine JF, Gueguen N, Boutet-Bouzamondo N, et al. Estrogen-related receptor alpha modulates lactate dehydrogenase activity in thyroid tumors. PLoS One. 2013; 8(3):e58683.

7). Lee MH, Lee SE, Kim DW, Ryu MJ, Kim SJ, Kim SJ, et al. Mitochondrial localization and regulation of BRAFV600E in thyroid cancer: a clinically used RAF inhibitor is unable to block the mitochondrial activities of BRAFV600E. J Clin Endocrinol Metab. 2011; 96(1):E19–30.

8). Herranz D, Maraver A, Canamero M, Gomez-Lopez G, Inglada-Perez L, Robledo M, et al. SIRT1 promotes thyroid carcinogenesis driven by PTEN deficiency. Oncogene. 2013; 32(34):4052–6.

9). Kweon KH, Lee CR, Jung SJ, Ban EJ, Kang SW, Jeong JJ, et al. Sirt1 induction confers resistance to etoposide-induced genotoxic apoptosis in thyroid cancers. Int J Oncol. 2014; 45(5):2065–75.

10). Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009; 460(7255):587–91.

11). Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008; 105(28):9793–8.

12). Herranz D, Munoz-Martin M, Canamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, et al. Sirt1 improves healthy ageing and protects from metabolic syndrome- associated cancer. Nat Commun. 2010; 1:3.

13). Fang Y, Nicholl MB. Sirtuin 1 in malignant transformation: friend or foe? Cancer Lett. 2011; 306(1):10–4.

15). Huffman DM, Grizzle WE, Bamman MM, Kim JS, Eltoum IA, Elgavish A, et al. SIRT1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007; 67(14):6612–8.

16). Bradbury CA, Khanim FL, Hayden R, Bunce CM, White DA, Drayson MT, et al. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia. 2005; 19(10):1751–9.

18). Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, et al. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008; 8(4):333–41.

19). Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008; 3(4):e2020.

20). Kabra N, Li Z, Chen L, Li B, Zhang X, Wang C, et al. SirT1 is an inhibitor of proliferation and tumor formation in colon cancer. J Biol Chem. 2009; 284(27):18210–7.

21). Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008; 32(1):11–20.

22). Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007; 27(24):8807–14.

23). Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010; 464(7285):121–5.
24). Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009; 119(9):2758–71.

25). Li S, Banck M, Mujtaba S, Zhou MM, Sugrue MM, Walsh MJ. p53-induced growth arrest is regulated by the mitochondrial SirT3 deacetylase. PLoS One. 2010; 5(5):e10486.

26). Ashraf N, Zino S, Macintyre A, Kingsmore D, Payne AP, George WD, et al. Altered sirtuin expression is associated with node-positive breast cancer. Br J Cancer. 2006; 95(8):1056–61.

27). Allison SJ, Milner J. SIRT3 is pro-apoptotic and participates in distinct basal apoptotic pathways. Cell Cycle. 2007; 6(21):2669–77.

28). Ford J, Jiang M, Milner J. Cancer-specific functions of SIRT1 enable human epithelial cancer cell growth and survival. Cancer Res. 2005; 65(22):10457–63.

29). Bell EL, Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell. 2011; 42(5):561–8.

30). Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011; 41(2):139–49.

Go to : 
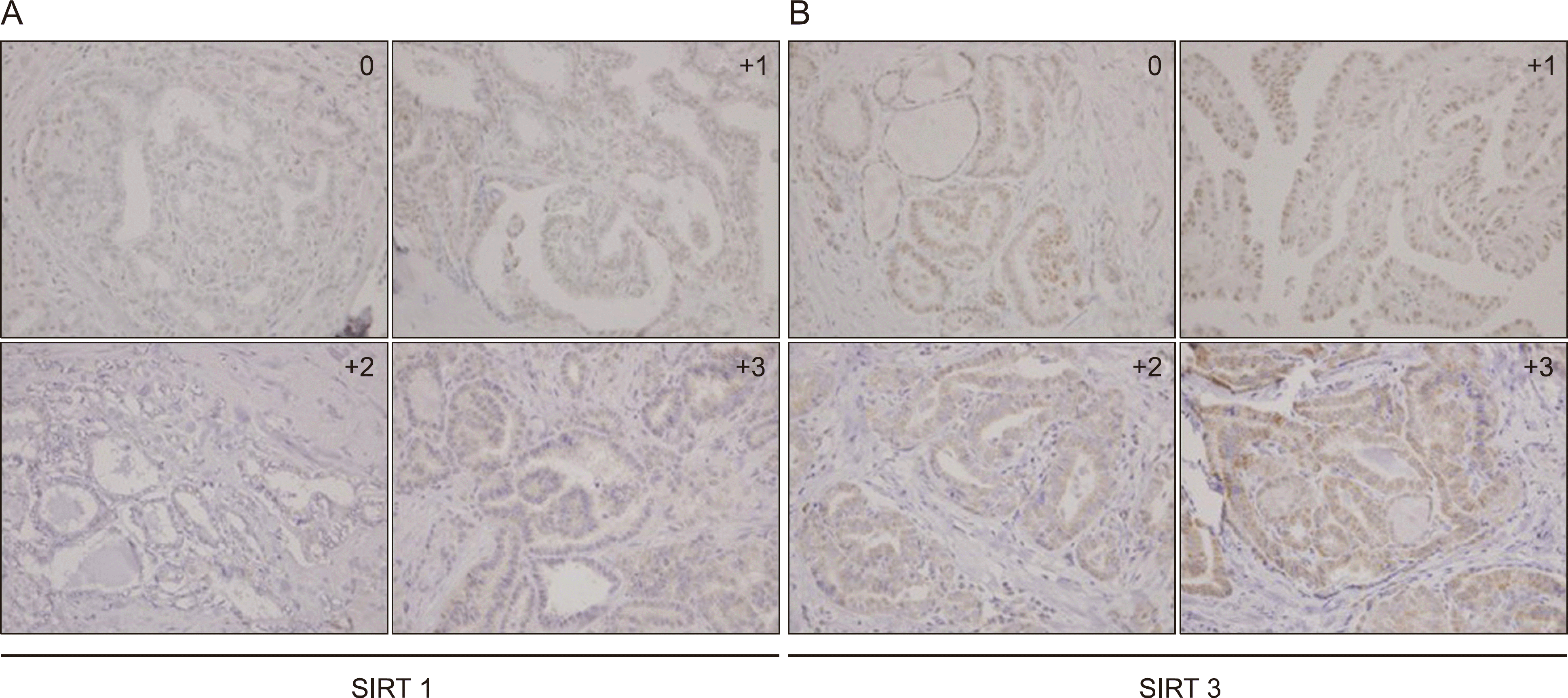 | Fig. 1.Immunohistochemical evaluation of sirtuin 1 (SIRT1) and sirtuin 3 (SIRT3) expression in papillary thyroid carcinoma (PTC) tissue. (A) Representative immunohistochemical images of SIRT1. (B) Representative immunohistochemical images of SIRT3. 0: no staining intensity, +1: weak staining intensity, +2: moderate staining intensity, +3: strong staining intensity (magnification ×100) |
Table 1.
Clinicopathologic parameters of patients (n=270)
Table 2.
Relationships between intensity of sirtuin 1 (SIRT1) staining and clinicopathological factors in 270 patients
| Variables | No. of patients | SIRT1 | |||
|---|---|---|---|---|---|
| Low (Grade 1 and 2) | High (Grade 3 and 4) | p value | |||
| Age, years | <45 | 104 | 66 | 38 | 0.411 |
| ≥45 | 166 | 97 | 69 | ||
| Gender | Male | 47 | 31 | 16 | 0.389 |
| Female | 223 | 132 | 91 | ||
| Tumor size | ≤1 cm | 110 | 61 | 49 | 0.171 |
| >1 cm | 160 | 102 | 58 | ||
| Multicentricity | No | 158 | 96 | 62 | 0.877 |
| Yes | 112 | 67 | 45 | ||
| Microscopic capsular invasion | No | 71 | 37 | 34 | 0.098 |
| Yes | 199 | 126 | 73 | ||
| Extrathyroid extension | No | 88 | 48 | 40 | 0.174 |
| Yes | 182 | 115 | 67 | ||
| Lymphovascular invasion | No | 63 | 31 | 32 | 0.039∗ |
| Yes | 207 | 132 | 75 | ||
| Lymph node metastasis | No | 116 | 58 | 58 | 0.002∗ |
| Yes | 154 | 105 | 49 | ||
| Central lymph node metastasis | No | 116 | 58 | 58 | 0.002∗ |
| Yes | 154 | 105 | 49 | ||
| Lateral lymph node metastasis | No | 222 | 122 | 100 | <0.001∗ |
| Yes | 48 | 41 | 7 | ||
| Locoregional recurrence | No | 233 | 139 | 94 | 0.547 |
| Yes | 37 | 24 | 13 | ||
Table 3.
Multivariate analysis of the relationship between SIRT1 staining and clinicopathologic factors
| Factors | Exp (β) | SE | 95.0% CI | p value |
|---|---|---|---|---|
| Lymphovascular invasion | 0.645 | 0.309 | (0.352, 1.182) | 0.156 |
| Lateral lymph node metastasis | 0.233 | 0.443 | (0.097. 0.555) | 0.001∗ |
| Central lymph node metastasis | 0.681 | 0.275 | (0.345, 0.849) | 0.162 |
Table 4.
Relationships between intensity of sirtuin 3 (SIRT3) staining and clinicopathological factors in 270 patients
| Variables | No. of patients | SIRT3 | |||
|---|---|---|---|---|---|
| Low (Grade 1 and 2) | High (Grade 3 and 4) | p value | |||
| Age, years | <45 | 104 | 52 | 52 | 0.175 |
| ≥45 | 166 | 69 | 97 | ||
| Gender | Male | 47 | 27 | 20 | 0.055 |
| Female | 223 | 94 | 129 | ||
| Tumor size | ≤1 cm | 110 | 54 | 56 | 0.241 |
| >1 cm | 160 | 67 | 93 | ||
| Multicentricity | No | 158 | 66 | 92 | 0.232 |
| Yes | 112 | 55 | 57 | ||
| Microscopic capsular invasion | No | 71 | 34 | 37 | 0.544 |
| Yes | 199 | 87 | 112 | ||
| Extrathryoid extension | No | 88 | 43 | 45 | 0.352 |
| Yes | 182 | 78 | 104 | ||
| Lymphovascular invasion | No | 63 | 26 | 37 | 0.518 |
| Yes | 207 | 95 | 112 | ||
| Lymph node metastasis | No | 116 | 48 | 68 | 0.325 |
| Yes | 154 | 73 | 81 | ||
| Central lymph node metastasis | No | 116 | 48 | 68 | 0.325 |
| Yes | 154 | 73 | 81 | ||
| Lateral lymph node metastasis | No | 222 | 95 | 127 | 0.151 |
| Yes | 48 | 26 | 22 | ||
| Locoregional recurrence | No | 233 | 111 | 122 | 0.019∗ |
| Yes | 37 | 10 | 27 | ||
Table 5.
Relationships between patterns of sirtuin staining intensity and clinicopathological factors
| Variables | SIRT1 low SIRT3 low (n=88) | SIRT1 low SIRT3 high (n=75) | SIRT1 high SIRT3 low (n=33) | SIRT1 high SIRT3 high (n=74) | p value | |
|---|---|---|---|---|---|---|
| Age, years | <45 | 40 | 26 | 12 | 26 | 0.444 |
| ≥45 | 48 | 49 | 21 | 48 | ||
| Gender | Male | 12 | 14 | 11 | 10 | 0.368 |
| Female | 76 | 43 | 40 | 64 | ||
| Tumor size | ≤1 cm | 35 | 26 | 19 | 30 | 0.168 |
| >1 cm | 53 | 49 | 14 | 44 | ||
| Multicentricity | No | 49 | 47 | 17 | 45 | 0.648 |
| Yes | 39 | 28 | 16 | 29 | ||
| Microscopic capsular invasion | No | 23 | 14 | 11 | 23 | 0.265 |
| Yes | 65 | 61 | 22 | 51 | ||
| Extrathryoid extension | No | 28 | 20 | 15 | 25 | 0.289 |
| Yes | 60 | 55 | 18 | 49 | ||
| Lymphovascular invasion | No | 17 | 14 | 9 | 23 | 0.214 |
| Yes | 71 | 61 | 24 | 51 | ||
| Lymph node metastasis | No | 30 | 28 | 18 | 40 | 0.025∗ |
| Yes | 58 | 47 | 15 | 34 | ||
| Central lymph node metastasis | No | 30 | 28 | 18 | 40 | 0.025∗ |
| Yes | 58 | 47 | 15 | 34 | ||
| Lateral lymph node metastasis | No | 64 | 58 | 31 | 69 | 0.001∗ |
| Yes | 24 | 17 | 2 | 5 | ||
| Locoregional recurrence | No | 79 | 60 | 32 | 62 | 0.072 |
| Yes | 9 | 15 | 1 | 12 | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download