Abstract
Background and Objectives
Hypermethylation of the tumor suppressor gene RASSF1A and activating mutation of BRAF gene have been recently reported in thyroid cancers. To investigate the role of these two epigenetic and genetic alterations in thyroid tumor progression, methylation of RASSF1A and BRAF mutation were examined in thyroid tumors.
Materials and Methods
During 2007 to 2017, 69 papillary carcinomas, 18 nodular hyperplasia, 3 follicular carcinomas, and 13 follicular adenomas were selected. The methylation-specific polymerase chain reaction (MSP) technique was used in detecting RASSF1A methylation and polymerase chain reaction (PCR)-single-stranded conformation polymorphism and sequencing were used for BRAF gene mutation study.
Results
The hypermethylation of the RASSF1A gene was found in 84.6%, 100% and 57.9% of follicular adenomas, follicular carcinomas, and papillary carcinomas, respectively. Nodular hyperplasia showed a hypermethylation in 33.3%. The BRAF mutation at V600E was found in 60.7% of papillary carcinoma and 27.0% of nodular hyperplasia, but none of follicular neoplasms. The BRAF mutation was correlated with the lymph node metastasis and MACIS clinical stage. There is an inverse correlation between RASSF1A methylation and BRAF mutation in thyroid lesions.
Figures and Tables
Fig. 1
The methylation status of the RASSF1A promoter region was analyzed by MSP; Methylation (m) and unmethylation-specific (u) primers were used. The methylation-specific product (93 bp) and unmethylation-specific product (105 bp) were resolved on 1.8% TBE gel. FA: follicular adenoma, FC: follicular carcinoma, NH: nodular hyperplasia, PC: papillary carcinoma

Fig. 2
The example of BRAF (exon 15) mutation was analyzed by PCR-SSCP (single-stranded conformation polymorphism) in papillary thyroid carcinoma. The arrow indicates the mutation band. M: mutation, N: normal
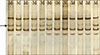
Fig. 3
Sequencing of the BRAF gene. Heterozygous missense mutation (T1796A/V600E) in exon 15. (A) Papillary carcinoma tissue and (B) nucleotide sequence in the corresponding normal tissue.

References
1. Ministry of Health and Welfare, Korea Cetral Cancer Registry, National Cancer Center. Anual report of cancer statistics in Korea in 2017.
2. Baylin SB. Mechanisms underlying epigenetically mediated gene silencing in cancer. Semin Cancer Biol. 2002; 12(5):331–337.

3. Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003; 63(7):1454–1457.
4. Moretti F, Nanni S, Pontecorvi A. Molecular pathogenesis of thyroid nodules and cancer. Baillieres Best Pract Res Clin Endocrinol Metab. 2000; 14(4):517–539.

5. Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003; 22(29):4578–4580.

6. Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003; 88(9):4393–4397.

8. Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000; 60(21):6116–6133.
9. Agathanggelou A, Honorio S, Macartney DP, Martinez A, Dallol A, Rader J, et al. Methylation associated inactivation of RASSF1A from region 3p21.3 in lung, breast and ovarian tumours. Oncogene. 2001; 20(12):1509–1518.

10. Burbee DG, Forgacs E, Zochbauer-Muller S, Shivakumar L, Fong K, Gao B, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst. 2001; 93(9):691–699.

11. Dammann R, Yang G, Pfeifer GP. Hypermethylation of the cpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001; 61(7):3105–3109.
12. Xing M, Cohen Y, Mambo E, Tallini G, Udelsman R, Ladenson PW, et al. Early occurrence of RASSF1A hypermethylation and its mutual exclusion with BRAF mutation in thyroid tumorigenesis. Cancer Res. 2004; 64(5):1664–1668.

13. Schagdarsurengin U, Gimm O, Hoang-Vu C, Dralle H, Pfeifer GP, Dammann R. Frequent epigenetic silencing of the CpG island promoter of RASSF1A in thyroid carcinoma. Cancer Res. 2002; 62(13):3698–3701.
14. Boltze C, Zack S, Quednow C, Bettge S, Roessner A, Schneider-Stock R. Hypermethylation of the CDKN2/p16INK4A promotor in thyroid carcinogenesis. Pathol Res Pract. 2003; 199(6):399–404.

15. Harada K, Toyooka S, Maitra A, Maruyama R, Toyooka KO, Timmons CF, et al. Aberrant promoter methylation and silencing of the RASSF1A gene in pediatric tumors and cell lines. Oncogene. 2002; 21(27):4345–4349.

16. Hawthorn L, Stein L, Varma R, Wiseman S, Loree T, Tan D. TIMP1 and SERPIN-A overexpression and TFF3 and CRABP1 underexpression as biomarkers for papillary thyroid carcinoma. Head Neck. 2004; 26(12):1069–1083.

17. Ito Y, Yoshida H, Tomoda C, Uruno T, Miya A, Kobayashi K, et al. S100A4 expression is an early event of papillary carcinoma of the thyroid. Oncology. 2004; 67(5-6):397–402.

18. Kato N, Tsuchiya T, Tamura G, Motoyama T. E-cadherin expression in follicular carcinoma of the thyroid. Pathol Int. 2002; 52(1):13–18.

19. Xing M, Usadel H, Cohen Y, Tokumaru Y, Guo Z, Westra WB, et al. Methylation of the thyroid-stimulating hormone receptor gene in epithelial thyroid tumors: a marker of malignancy and a cause of gene silencing. Cancer Res. 2003; 63(9):2316–2321.
20. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 417(6892):949–954.

21. Brose MS, Volpe P, Feldman M, Kumar M, Rishi I, Gerrero R, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002; 62(23):6997–7000.
22. Naoki K, Chen TH, Richards WG, Sugarbaker DJ, Meyerson M. Missense mutations of the BRAF gene in human lung adenocarcinoma. Cancer Res. 2002; 62(23):7001–7003.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download