Abstract
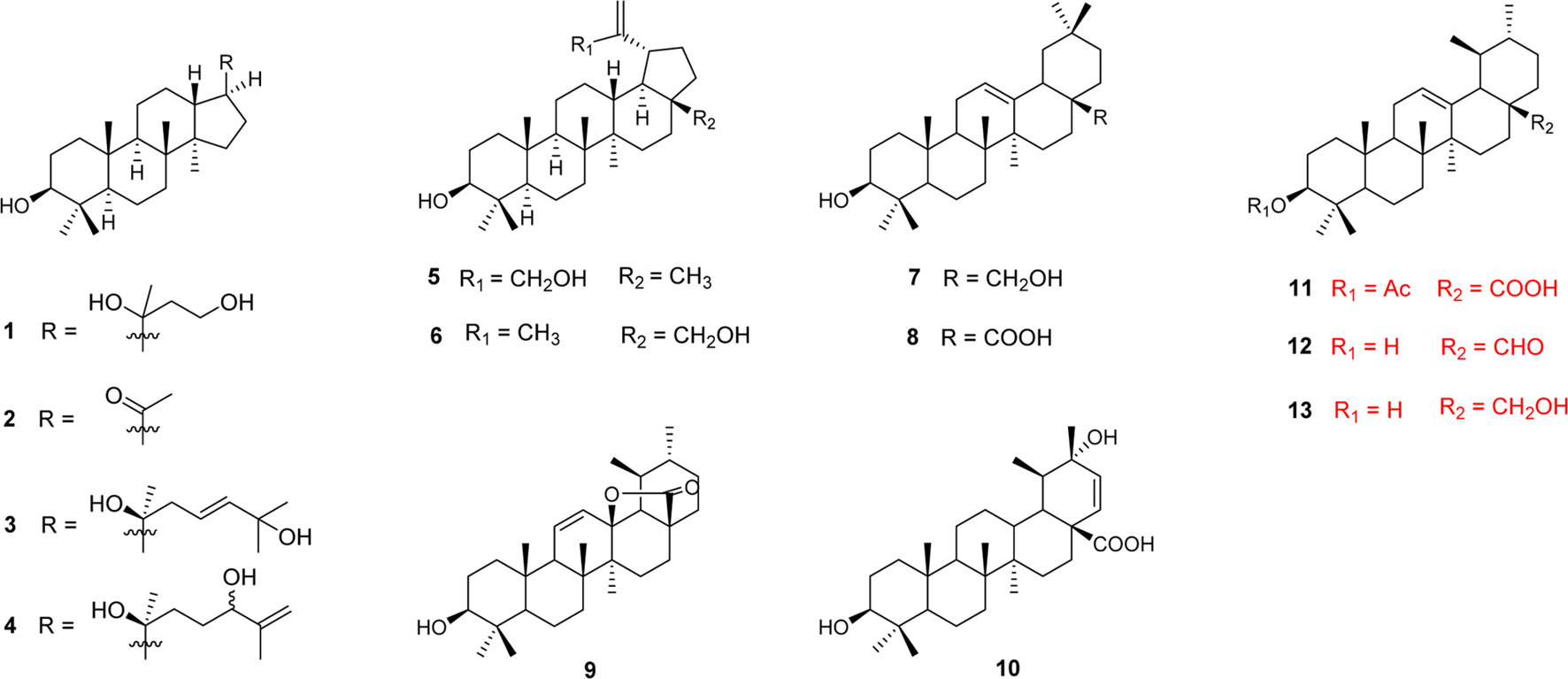
Medicinal plants are potential sources of anticancer agents screening. A large number of phytochemicals, including triterpenoids, have been reported to have significant cytotoxic effects on cancer cells. From the fruits of Ligustrum japonicum Thunb., thirteen triterpenoids (1 – 13) were isolated and evaluated for their cytotoxic activity against Hela and HL-60 cells. As results, 8 (oleanolic acid) showed significant effects on Hela with IC50 values of 5.5 µM, and moderate effects on HL-60 cells with IC50 values of 55.9 µM. Meanwhile, 10 (oleanderic acid) and 11 (3β-acetoxy-urs-12-en-28-oic acid) exhibited moderate inhibitory effects on Hela with IC50 value of 55.0 and 68.8 µM, respectively. Moreover, 10 showed cytotoxic effect on HL-60 cell line with IC50 value of 63.9 µM. To our knowledge, this is the first report that oleanderic acid was isolated from L. japonicum and investigated in cytotoxic effects on Hela and HL-60 cells.
Go to : 
References
(1). Jensen S. R.., Franzyk H.., Wallander E.Phytochemistry. 2002. 60:213–231.
(2). Ngo Q. -M.T.., Lee H. S.., Nguyen V. T.., Kim J. A.., Woo M. H.., Min B. S.Phytochemistry. 2017. 141:147–155.
(3). Chudzik M.., Korzonek-Szlacheta I.., Król W.Molecules. 2015. 20:1610–1625.
(4). Taher M.., Aminuddin A.., Susanti D.., Aminudin N. I.., On S.., Ahmad F.., Hamidon H.Nat. Prod. Sci. 2016. 22:122–128.
(5). Tanaka R.., Matsuda M.., Matsunaga S.Phytochemistry. 1987. 26(3365–3366):. 3365–3366.3365–3366.
(6). Pakhathirathien C.., Karalai C.., Ponglimanont C.., Subhadhirasakul S.., Chantrapromma K. J.Nat. Prod. 2005. 68:1787–1789.
(7). Chakrabartty T.., Poddar G.., Pyrek J. S.Phytochemistry. 1982. 21:1814–1816.
(8). Fuchino H.., Satoh T.., Tanaka N.Chem. Pharm. Bull. 1996. 44:1748–1753. . 1748–1753.
(9). Nomura M.., Tokoroyama T.., Kubota T.Phytochemistry. 1981. 20:1097–1104. . 1097–1104.
(10). Seebacher W.., Simic N.., Weis R.., Saf R.., Kunert O.Magn. Reson. Chem. 2003. 41:636–638.
(11). Al Musayeib N. M.., Mothana R. A.., Gamal A. A. E.., Al-Massarani S. M.., Maes L.Molecules. 2013. 18:9207–9218.
(12). Fu L.., Zhang S.., Li N.., Wang J.., Zhao M.., Sakai J.., Hasegawa T.., Mitsui T.., Kataoka T.., Oka S.., Kiuchi M.., Hirose K.., Ando M. J.Nat. Prod. 2005. 68:198–206.
(13). Batra A.., Sastry V. G.Pteridines. 2013. 24:191–199.
(14). Hota R. K.., Bapuji M.Phytochemistry. 1994. 35:1073–1074.
(15). Liao C. R.., Kuo Y. H.., Ho Y. L.., Wang C. Y.., Yang C. S.., Lin C. W.., Chang Y. S.Molecules. 2014. 19:9515–9534.
(16). Butruille D.., Dominguez X. A.Tetrahedron Lett. 1974. 15:639–642.
(17). Rouf A. S. S.., Ozaki Y.., Rashid M. A.., Rui J.Phytochemistry. 2001. 56:815–818.
(18). Zhu Y. Y.., Huang H. Y.., Wu Y. L.Mol. Med. Rep. 2015. 12:5012–5018.
(19). Akihisa T.., Kamo S.., Uchiyama T.., Akazawa H.., Banno N.., Taguchi Y.., Yasukawa K. J.Nat. Med. 2006. 60:331–333.
(20). Chiang Y. M.., Chang J. Y.., Kuo C. C.., Chang C. Y.., Kuo Y. H.Phytochemistry. 2005. 66:495–501.
Go to : 
Table 1.
Cytotoxic activities of compounds 1 – 13 against Hela and HL-60 cell lines.
| Compds | IC50, µMa | Compds | IC50, µMa | ||
|---|---|---|---|---|---|
| Hela | HL-60 | Hela | HL-60 | ||
| 1 | > 100 | > 100 | 8 | 5.5 ± 0.7 | 55.9 ± 1.2 |
| 2 | > 100 | > 100 | 9 | > 100 | > 100 |
| 3 | > 100 | > 100 | 10 | 55.0 ± 2.5 | 63.9 ± 2.8 |
| 4 | > 100 | > 100 | 11 | 68.8 ± 5.1 | > 100 |
| 5 | > 100 | > 100 | 12 | > 100 | > 100 |
| 6 | > 100 | > 100 | 13 | > 100 | > 100 |
| 7 | > 100 | > 100 | Adriamycinb | 0.67 ± 0.5 | 0.24 ± 0.06 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download