Abstract
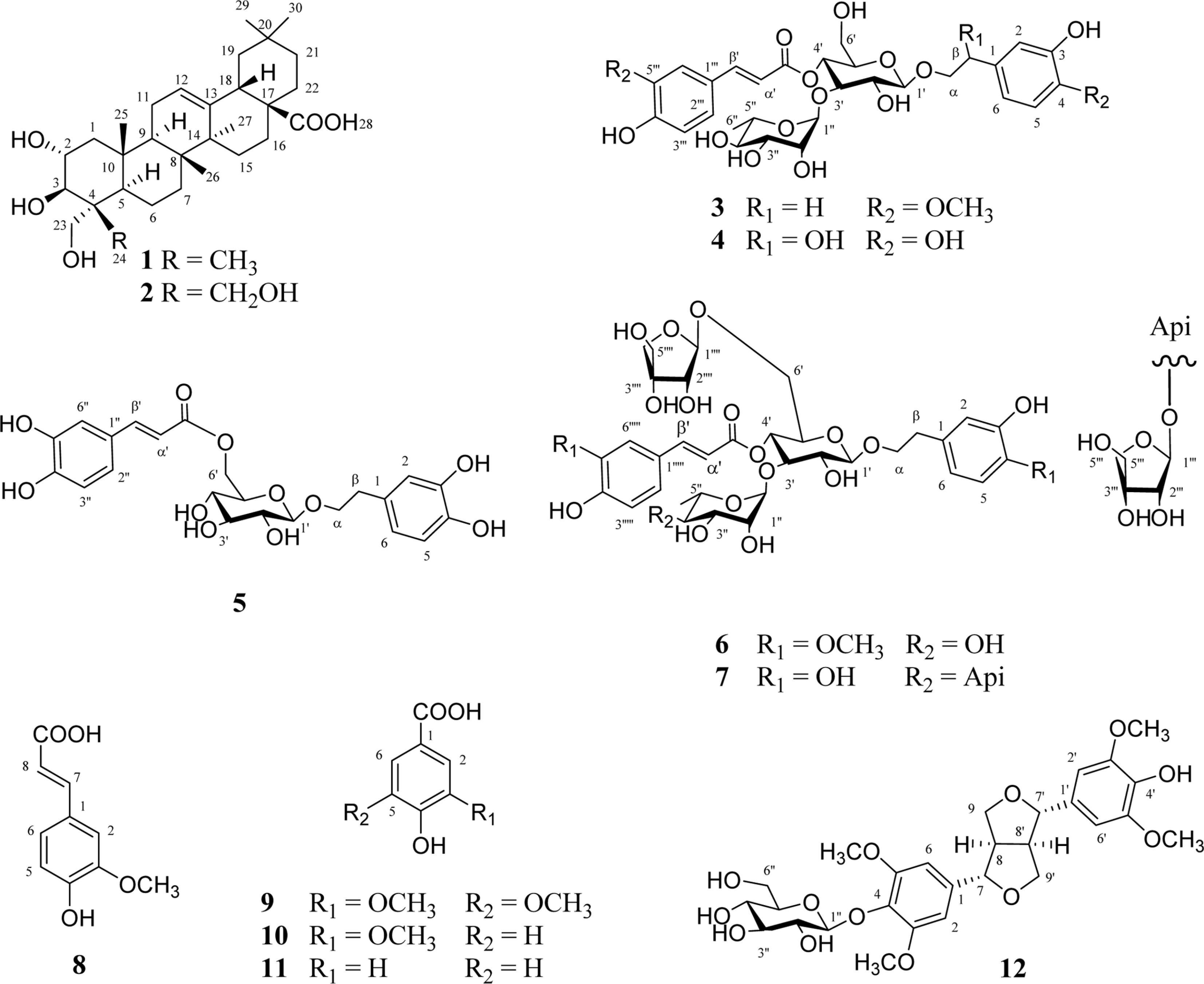
Two triterpenoids, arjunolic acid (1), belleric acid (2), five phenylethanoids, martynoside (3), orobanchoside (4), 3,4-dihydroxyphenethylalcohol-6-O-caffeoyl-β-D-glucoside (5), leucosceptoside B (6), lunarii-folioside (7), four phenolic acids, ferulic acid (8), syringic acid (9), vanillic acid (10), 4-hydroxybenzoic acid (11), and one lignan, (+)-syringaresinol-β-D-glucoside (12), were isolated from the roots of P. umbrosa. All isolated compounds were explored for their antioxidant potential in the DPPH and ABTS assays. In DPPH assay, compound 5 showed high antioxidant capacity. Compounds 3, 4, 6, and 7 displayed considerable antioxidant activities. In addition, compounds 5–7 exhibited potential antioxidant capacities in the ABTS assay.
Go to : 
References
(1). Shen L.., Ji H. F.Trends Food Sci. Technol. 2017. 68:51–55.
(2). Bennett L. L.., Rojas S.., Seefeldt T. J.Exp. Clin. Med. 2012. 4:215–222.
(3). Lee D.., Kim Y. S.., Song J.., Kim H. S.., Lee H. J.., Guo H.., Kim H.Molecules. 2016. 21:461.
(4). Zhang Y.., Wang Z. Z. J.Pharm. Biomed. Anal. 2008. 47:213–217.
(5). Liu P.., Deng R.., Duan H.., Yin W.Zhongguo Zhong Yao Za Zhi. 2009. 34:867–870.
(6). Skrzypek Z.., Wysoki ska H.., wiatek L.., Wróblewski A. E. J.Nat. Prod. 1999. 62:127–129.
(7). Nishibe S.., Tamayama Y.., Sasahara M.., Andary C.Phytochemistry. 1995. 38:741–743.
(8). Matsumoto M.., Koga S.., Shoyama Y.., Nishioka I.Phytochemistry. 1987. 26:3225–3227.
(9). Saraco lu., Harput Ü.., Çaliþ Turk. J. Chem. 2002. 26:133–142.
(11). Sajjadi S. E.., Shokoohinia Y.., Moayedi N. S. Jundishapur J.Nat. Pharm. Prod. 2012. 7:159–162.
(12). Ngan L. T. M.., Moon J. K.., Shibamoto T.., Ahn Y. J. J.Agric. Food Chem. 2012. 60:9062–9073.
(13). Nguyen D. H.., Zhao B. T.., Le D. D.., Kim K. Y.., Kim Y. H.., Yoon Y. H.., Woo K. S.., Ko J. Y.., Woo M. H.Nat. Prod. Sci. 2016. 22:140–145.
(14). Shahat A. A.., Abdel-Azim N. S.., Pieters L.., Vlietinck A. J.Fitoterapia. 2004. 75:771–773.
(15). Manna P.., Sinha M.., Sil P. C.Arch. Toxicol. 2008. 82:137–149.
(16). Pendota S. C.., Aderogba M. A.., Van Staden J. S.Afr. J. Bot. 2015. 96:91–93.
(17). Papoutsi Z.., Kassi E.., Mitakou S.., Aligiannis N.., Tsiapara A.., Chrousos G. P.., Moutsatsou P. J.Steroid Biochem. Mol. Biol. 2006. 98:63–71.
(18). Yin Z.., Zhang W.., Feng F.., Zhang Y.., Kang W.Food Science and Human Wellness. 2014. 3:136–174.
(19). Yamazaki T.., Shimosaka S.., Sasaki H.., Matsumura T.., Tukiyama T.., Tokiwa T.Toxicol. In Vitro. 2007. 21:1530–1537.
(20). Kumar N.., Pruthi V.Biotechnol. Rep. 2014. 4:86–93.
(21). Karthik G.., Angappan M.., Vijayakumar A.., Natarajapillai S.Biomed. Prev. Nutr. 2014. 4:203–208.
(22). Calixto-Campos C.., Carvalho T. T.., Hohmann M. S. N.., Pinho-Ribeiro F. A.., Fattori V.., Manchope M. F.., Zarpelon A. C.., Baracat M. M.., Georgetti S. R.., Casagrande R.., Verri W. A.Jr. J. Nat. Prod. 2015. 78:1799–1808.
(23). Cho J. Y.., Moon J. H.., Seong K. Y.., Park K. H.Biosci. Biotehnol. Biochem. 1998. 62:2273–2276.
Go to : 
Table 1.
The antioxidant capacities of isolated compounds (1–12) in DPPH and ABTS assay




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download