Abstract
Artemisia capillaris Thunb. (Compositae) is a native herb of East Asian countries and has used for the treatment of jaundice, high liver fever, and digestive diseases for a long time, as well as being developed as the source of herbal preparations until now. The major components from A. capillaris were chlorogenic acid (1) and its derivatives substituted with caffeoyl moieties, such as 3,5-dicaffeoylquinic acid (2) and 4,5-dicaffeoylquinic acid (3), and coumarins, such as scoparone. In the study, four compounds, chlorogenic acid, 3,5-dicaffeoylquinic acid, 4,5-dicaffeoylquinic acid and scoparone (4) in the 70% ethanolic extract of A. capillaris were simultaneously determined by using HPLC-UVD system. This method was validated with the terms of linearity, precious and accuracy according to ICH guidelines. The developed method was successfully applied for the quantitative analysis of Artemisia genus, A. capillaris, A. iwayomogi, A. princeps, and A. argyi, distributed in Korea.
Go to : 
References
(1). Jang E.., Kim B. J.., Lee K. T.., Inn K. S.., Lee J. H.Evid. Based Complement. Alternat. Med. 2015. 2015:728137.
(2). Islam M. N.., Choi R. J.., Jung H. A.., Oh S. H.., Choi J. S.Arch. Pharm. Res. 2016. 39:340–349.
(3). Choi M. K.., Han J. M.., Kim H. G.., Lee J. S.., Lee J. S.., Wang J. H.., Son S. W.., Park H. J.., Son C. G. J.Ethnopharmacol. 2013. 147:662–670.
(4). Chun K. S.., Kang J. Y.., Kim O. H.., Kang H.., Surh Y. J. J.Environ. Pathol. Toxicol. Oncol. 2002. 21:131–139.
(5). Koo B. S.., Lee W. C.., Chang Y. C.., Kim C. H.Phytother. Res. 2004. 18:142–148.
(6). Ha H.., Lee H.., Seo C. S.., Lim H. S.., Lee J. K.., Lee M. Y.., Shin H.BMC Complement. Altern. Med. 2014. 14:100.
(7). Park K. M.., Li Y.., Kim B.., Zhang H.., Hwangbo K.., Piao D. G.., Chi M. J.., Woo M. H.., Choi J. S.., Lee J. H.., Moon D. C.., Chang H. W.., Kim J. R.., Son J. K.Arch. Pharm. Res. 2012. 35:2153–2162.
(8). Okuno I.., Uchida K.., Nakamura M.., Sakurawi K.Chem. Pharm. Bull. 1988. 36:769–775.
(9). Jang S. I.., Kim Y. J.., Lee W. Y.., Kwak K. C.., Baek S. H.., Kwak G. B.., Yun Y. G.., Kwon T. O.., Chung H. T.., Chai K. Y.Arch. Pharm. Res. 2005. 28:203–208.
(10). Cho D. Y.., Ko H. M.., Kim J.., Kim B. W.., Yun Y. S.., Park J. I.., Ganesan P.., Lee J. T.., Choi D. K.Molecules. 2016. 21:, E1718.
(11). Jang S. I.., Kim Y. J.., Kim H. J.., Lee J. C.., Kim H. Y.., Kim Y. C.., Yun Y. G.., Yu H. H.., You Y. O.Life Sci. 2006. 78:2937–2943.
(12). Tan X. J.., Li Q.., Chen X. H.., Wang Z. W.., Shi Z. Y.., Bi K. S.., Jia Y. J.Pharm. Biom. Anal. 2008. 47:847–853.
Go to : 
 | Fig. 1.Structures of four standards, chlorogenic acid (1), 3,5–dicaffeoylquinic acid (2), 4,5–dicaffeoylquinic acid (3) and scoparone (4), from A. capillaris. |
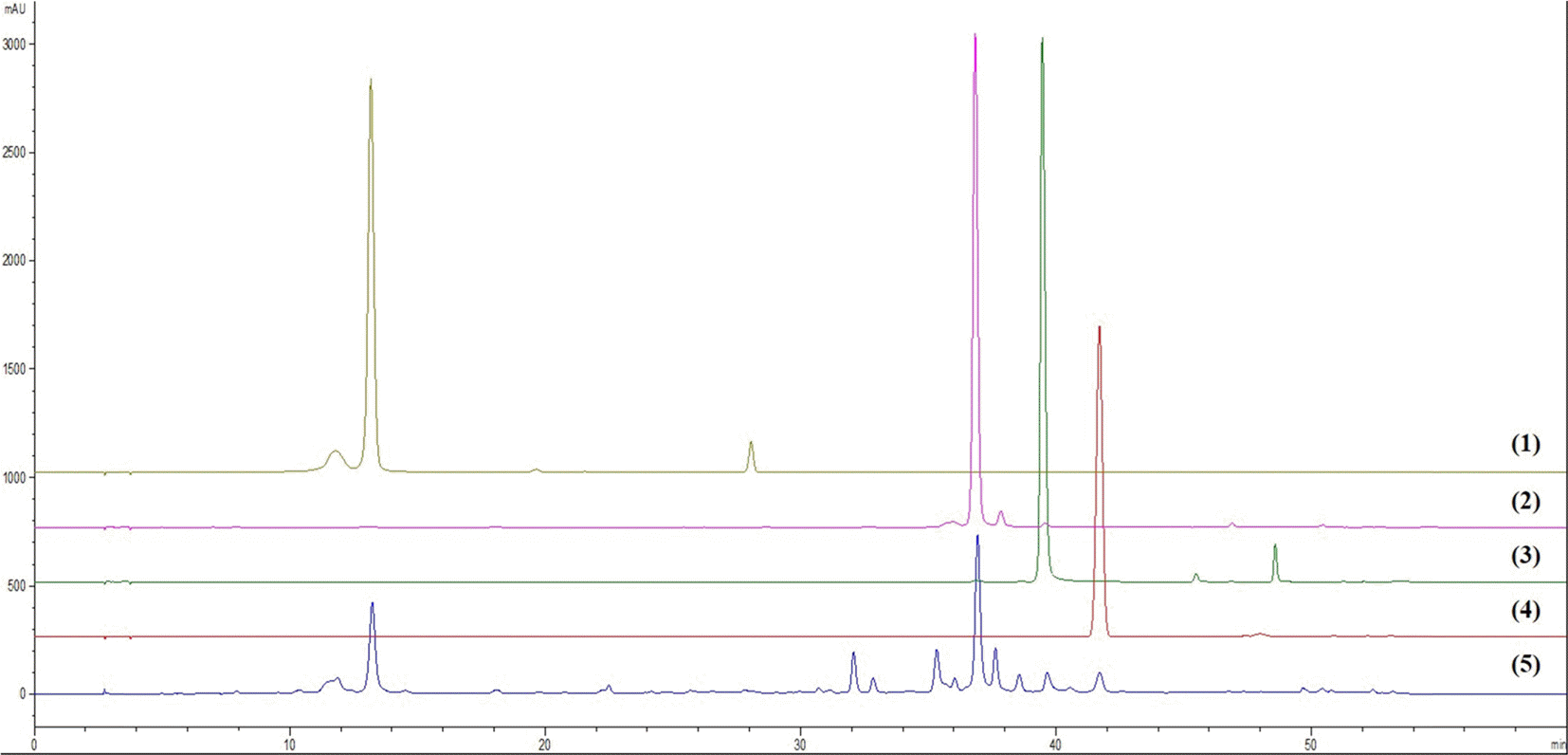 | Fig. 2.HPLC chromatogram of four standards, chlorogenic acid (1), 3,5–dicaffeoylquinic acid (2), 4,5–dicaffeoylquinic acid (3), scoparone (4), and the 70% ethanolic extract of A. capillaris (5). |
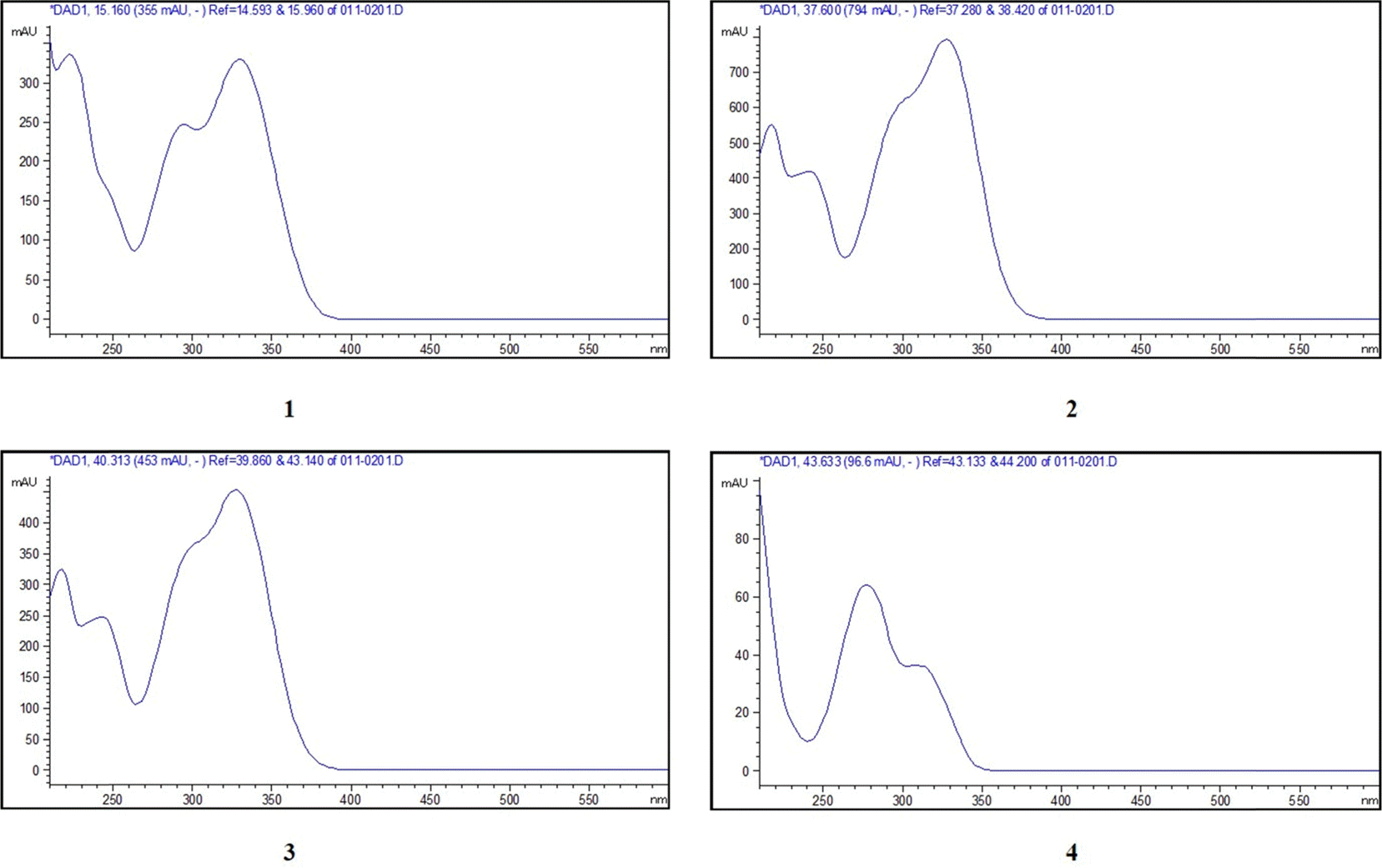 | Fig. 3.UV spectra of four standards, chlorogenic acid (1), 3,5–dicaffeoylquinic acid (2), 4,5–dicaffeoylquinic acid (3) and scoparone (4). |
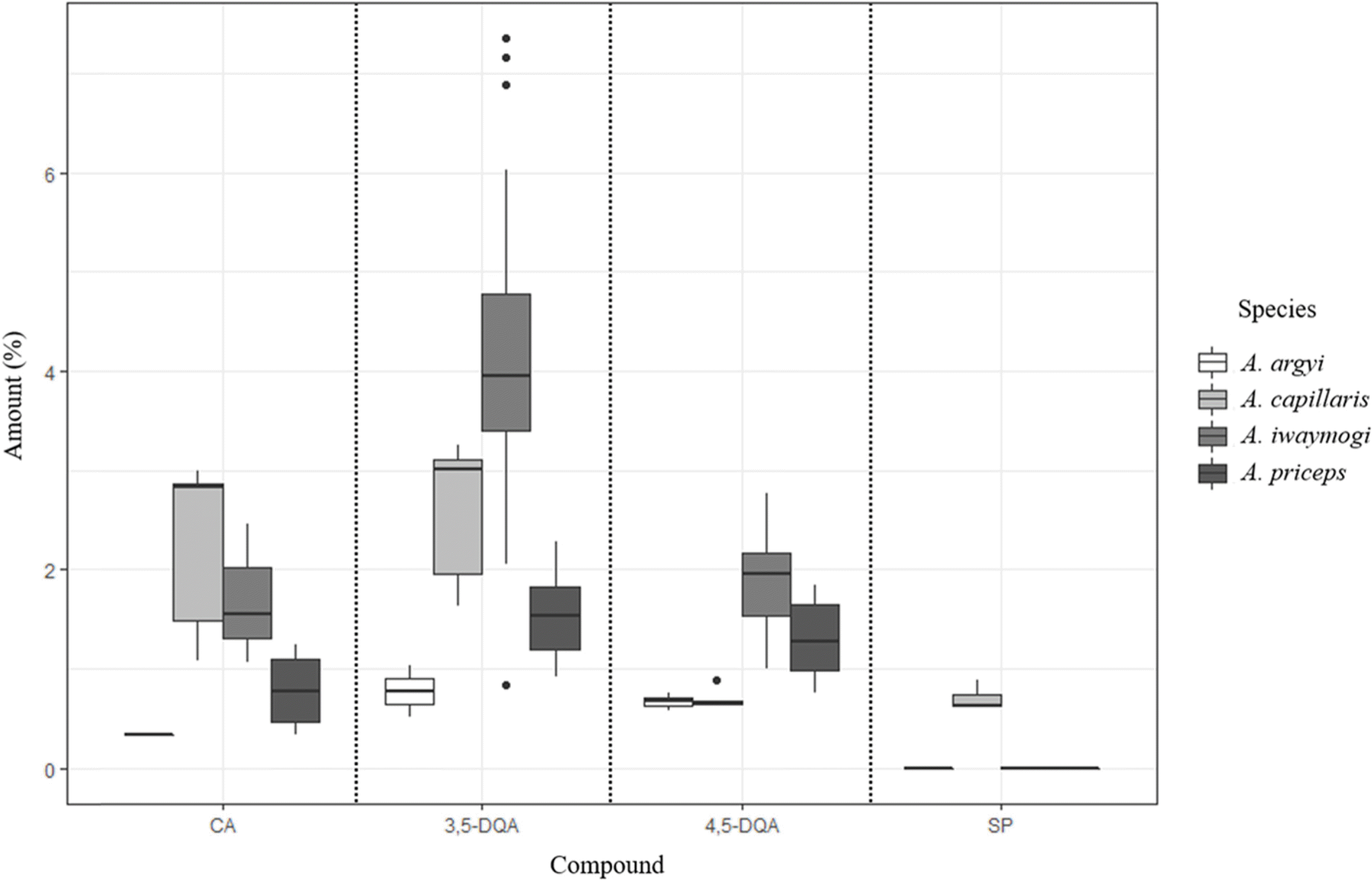 | Fig. 4.Boxplot of the content of four standards in thirty-one Artemisia genus samples, chlorogenic acid (1), 3,5–dicaffeoylquinic acid (2), 4,5–dicaffeoylquinic acid (3) and scoparone (4). |
Table 1.
Calibration curves for LOD and LOQ of A. capillaris
| Compounds | Retention time | Linear regression equation | R2 | LOD (µg) LOQ (µg) |
|---|---|---|---|---|
| CAa | 13.2 | y = 25041x − 92.957 | 0.9997 | 0.0021 0.0063 |
| 3,5-DQA | 36.9 | y = 36831x − 162.99 | 0.9999 | 0.0029 0.0087 |
| 4,5-DQA | 39.5 | y = 29271x − 139.2 | 0.9999 | 0.0036 0.0110 |
| SP | 41.7 | y = 27270x − 29.067 | 0.9999 | 0.0006 0.0017 |
Table 2.
Precision and accuracy data of standard in A. capillaris
| Compounds | Preccision | Accuracy | |||||
|---|---|---|---|---|---|---|---|
| Intra day | Inter day | Spiked amount (µg/g) | Accuracy (%) | RSD (%) | |||
| amount (µg/g) | RSD (%) | amount (µg/g) | RSD (%) | ||||
| 3.365 | 4.499 | 0.659 | 3.218 | 3.508 | 95.92 | 4.50 | |
| CAa | 3.072 | 1.409 | 1.489 | 1.420 | 3.000 | 102.41 | 1.41 |
| 2.488 | 0.090 | 2.922 | 2.762 | 2.500 | 99.54 | 0.09 | |
| 3.347 | 1.796 | 0.600 | 0.675 | 3.523 | 95.02 | 1.80 | |
| 3,5-DQA | 2.988 | 1.839 | 1.422 | 5.617 | 3.023 | 98.87 | 1.84 |
| 2.455 | 1.501 | 3.002 | 2.573 | 2.523 | 97.33 | 1.50 | |
| 0.746 | 0.966 | 0.143 | 1.276 | 0.806 | 92.52 | 0.97 | |
| 4,5-DQA | 0.525 | 0.691 | 0.304 | 5.096 | 0.556 | 94.38 | 0.69 |
| 0.389 | 1.208 | 0.618 | 5.480 | 0.406 | 95.75 | 1.21 | |
| 0.806 | 1.487 | 0.132 | 0.934 | 0.788 | 102.27 | 1.49 | |
| SP | 0.560 | 0.884 | 0.318 | 6.099 | 0.538 | 104.07 | 0.88 |
| 0.406 | 1.019 | 0.625 | 4.106 | 0.388 | 104.65 | 1.02 | |
Table 3.
The amounts of four standards, CA, 3,5-DQA, 4,5-DQA and SP from Artemisia genus
| Mean ± SD (µg/g) | ||||
|---|---|---|---|---|
| CAa | 3,5-DQA | 4,5-DQA | SP | |
| AC-1b | 2.83 ± 0.07 | 3.11 ± 0.05 | 0.64 ± 0.01 | 0.61 ± 0.05 |
| AC-2 | 2.99 ± 0.06 | 3.25 ± 0.07 | 0.65 ± 0.01 | 0.63 ± 0.07 |
| AC-3 | 2.86 ± 0.07 | 3.01 ± 0.08 | 0.62 ± 0.01 | 0.63 ± 0.01 |
| AC-4 | 1.49 ± 0.03 | 1.95 ± 0.03 | 0.89 ± 0.01 | 0.89 ± 0.01 |
| AC-5 | 1.08 ± 0.06 | 1.64 ± 0.07 | 0.67 ± 0.02 | 0.74 ± 0.02 |
| AI-1 | 2.18 ± 0.01 | 4.00 ± 0.01 | 2.06 ± 0.01 | n.d.c |
| AI-2 | 1.96 ± 0.06 | 6.03 ± 0.17 | 2.77 ± 0.05 | n.d |
| AI-3 | 1.67 ± 0.03 | 4.18 ± 0.13 | 1.42 ± 0.02 | n.d |
| AI-4 | 1.39 ± 0.03 | 2.05 ± 0.04 | 1.00 ± 0.01 | n.d |
| AI-5 | 1.06 ± 0.02 | 3.17 ± 0.06 | 2.01 ± 0.02 | n.d |
| AI-6 | 1.47 ± 0.02 | 3.47 ± 0.09 | 2.45 ± 0.04 | n.d |
| AI-7 | 1.10 ± 0.00 | 3.07 ± 0.00 | 1.08 ± 0.01 | n.d |
| AI-8 | 1.34 ± 0.04 | 3.73 ± 0.09 | 2.49 ± 0.04 | n.d |
| AI-9 | 1.17 ± 0.02 | 0.84 ± 0.02 | 1.75 ± 0.02 | n.d |
| AI-10 | 1.22 ± 0.05 | 3.81 ± 0.08 | 2.59 ± 0.03 | n.d |
| AI-11 | 2.36 ± 0.06 | 7.17 ± 0.01 | 1.93 ± 0.00 | n.d |
| AI-12 | 2.46 ± 0.02 | 7.35 ± 0.05 | 2.07 ± 0.01 | n.d |
| AI-13 | 2.40 ± 0.01 | 6.88 ± 0.03 | 1.97 ± 0.01 | n.d |
| AI-14 | 1.49 ± 0.04 | 3.89 ± 0.05 | 1.47 ± 0.01 | n.d |
| AI-15 | 1.62 ± 0.03 | 4.36 ± 0.07 | 1.66 ± 0.02 | n.d |
| AI-16 | 1.71 ± 0.08 | 4.15 ± 0.07 | 1.56 ± 0.02 | n.d |
| AP-1 | 1.08 ± 0.06 | 1.93 ± 0.01 | 1.84 ± 0.01 | n.d |
| AP-2 | 0.33 ± 0.01 | 1.00 ± 0.01 | 0.99 ± 0.00 | n.d |
| AP-3 | 1.25 ± 0.07 | 1.80 ± 0.01 | 1.74 ± 0.00 | n.d |
| AP-4 | 0.54 ± 0.03 | 0.92 ± 0.02 | 1.11 ± 0.02 | n.d |
| AP-5 | 1.02 ± 0.02 | 1.62 ± 0.04 | 1.45 ± 0.02 | n.d |
| AP-6 | 1.15 ± 0.09 | 2.28 ± 0.09 | 1.61 ± 0.05 | n.d |
| AP-7 | 0.49 ± 0.00 | 1.45 ± 0.03 | 0.98 ± 0.01 | n.d |
| AP-8 | 0.40 ± 0.01 | 1.25 ± 0.01 | 0.75 ± 0.01 | n.d |
| AA-1 | 0.32 ± 0.01 | 0.52 ± 0.01 | 0.58 ± 0.01 | n.d |
| AA-2 | 0.35 ± 0.01 | 1.03 ± 0.03 | 0.76 ± 0.01 | n.d |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download