Abstract
Background
Procollagen type I N-terminal propeptide (PINP) is one of the most clinically useful bone formation biomarkers. Therefore, the purpose of this study was to independently evaluate the performance of automated total PINP assay and established age- and gender-specific reference intervals for PINP in healthy Korean population.
Methods
The imprecision, linearity, and detection capability of Elecsys total PINP assay was determined and reference interval was established using 599 serums from Korean population with normal bone mineral densities based on bone densitometry. Age groups were divided into 20s, 30s, 40s, 50s, 60s and over.
Results
Elecsys total PINP had excellent performance in imprecision, linearity, and detection capability. When partitioning age groups in Korean male and female populations, there was significant difference in total PINP between different age groups. In male populations, PINP level was decreased with increasing age, then it remained steady after middle-age. In female populations, there was a decreasing tendency similar to that in the male population with a sharp increase in the 50 to 59 age group.
The World Health Organization defines osteoporosis as a systemic skeletal disease characterized by a decrease in bone mass and an abnormality of the microstructure, resulting in a weakened bone that is liable to break.[1] The prevalence rate of osteoporosis in South Korea is estimated to increase by 7.4% every year from 1.4 million in 2008 to 1.96 million in 2012.[2]
So far, bone mineral density (BMD) is commonly used to diagnose osteoporosis by measuring dual energy X-ray absorptiometry (DXA).[3] However, monitoring of bone turnover markers (BTM) is necessary to observe the effect of osteoporosis treatment.[4] BTM has been developed to significantly improve understanding of the onset of osteoporosis and the understanding of fast bone losers. They are generally divided into bone resorption markers, bone formation markers and osteoclast control proteins. Biochemical BTM has been used for many years with attractive features including easy specimens and various assays that complement the radiological evaluation of patients and their implementation in clinical practice has helped to make optimal treatment choices.[567]
Procollagen type I N-terminal propeptide (PINP) is one of the precursor peptides removed from procollagen type I to produce type I collagen and considered as a marker of collagen type I synthesis.[8] PINP concentration is using marker to determine the efficacy of anti-resorptive treatment in osteoporosis patients and recognized as one of the most important BTMs by Joint International Osteoporosis Foundation-International Federation of Clinical Chemistry and Laboratory Medicine Bone Marker Standards Working Group.[910]
The establishment of reference intervals of PINP as bone forming biochemical marker is mandatory for clinical use.[11] Although reference intervals of PINP for populations in different countries were reported,[111213] there was no information about the reference ranges of serum PINP levels in South Korean Population.
Therefore, the purpose of this study was to establish age-related serum reference intervals for PINP values in the South Korean Population as determined by automated methods.
Blood samples were collected at 3 medical centers (Chung-Ang University Hospital, Gyeongsang National University Hospital, and Jeju National University Hospital) between September 2015 and March 2016. To establish age-specific reference range of PINP.
A total of 578 blood samples were collected from healthy adults (208 males and 370 females) who visited medical centers for routine health check-up. Characteristics of the male and female cohort are shown in Table 1. All participants were healthy adults with normal BMD based on DXA. This study was approved by Institutional Review Board (IRB) of Chung-Ang University Hospital, Gyeongsang National University Hospital, and Jeju National University Hospital. Informed consent was obtained from participants. The independent ethics committee of Chung-Ang University Hospital proved approval for the study to be performed (IRB no. C2016057 [1800]).
Serum PINP concentrations were measured using Elecsys total PINP assay (Roche Diagnostics, Mannheim, Germany) on an E170 module immunology analyzer (Roche Diagnostics). This assay is a 2-site assay using monoclonal antibodies for the detection of intact human PINP originated from 2nd-trimester human amniotic fluid. This assay is known to be able to detect both intact monomeric and trimetric forms of PINP.[14] All samples were measured with a single lot of assay agent following laboratory quality control procedure according to the manufacturer's specification. Blood samples for PINP measurement were collected in a consistent fashion following an overnight fast during the morning between 7:30 am and 10:00 am.
Linearity was assessed by mixing specimens with 2 different concentrations of PINP. Specimens with high or low P1NP concentration were mixed to make 5 P1NP concentration intervals. Samples were analyzed in triplicate samples of serum. Results of 5 mixed samples were compared to expected values.
Based on Clinical and Laboratory Standards Institute (CLSI) guideline EP05-A3,[15] each sample was measured on 5 consecutive days (2 runs per day, 2 replicates per run). Two different quality control samples at different concentrations and 5 human serum samples from normal healthy population were used for this evaluation.
According to CLSI guideline EP17-A2,[16] we investigated the detection capability of the PINP assay. At first, we measured normal saline in 60 replicates to establish the limit of blank (LoB; the highest measurement result likely to be observed for a blank sample). Then, specimens with the PINP value of near 5 µg/L was described as the lower detection limit based on manufacture's specifications. They were analyzed for 5 days (5 replicates) in order to determine the limit of detection (LoD).
All statistical analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA) and Microsoft Excel 2010 software (Microsoft Corporation, Redmond, WA, USA). Associations between measured PINP values and expected PINP values were assessed by linear regression. The repeatability and within-laboratory precision with 95% confidence intervals were calculated by analysis of variance. LoB and LoD were determined by parametric analysis with classical approach described in CLSI guideline.[16]
For the establishment of reference intervals, Tukey's test was employed to eliminate outliers. Kolmogorov-Smirnov test was then performed to assess whether PINP results of subpopulations were normal distribution, respectively. If the distribution of results was not Gaussian, logarithmic transformation was done to normalize the distribution. The geometric mean with 95% confidence interval, 2.5th, 97.5th percentiles, and interquartile range (IQR; 25–75%) were calculated for subpopulations separated into age groups by decades. Age-related reference interval was suggested as the central 95% of the distribution. Mann-Whitney test was used to compare PINP levels between 2 subpopulations. If more than 3 subpopulations were compared, Kruskal-Wallis test was used.
Results of regression analysis between measured values of mixed samples and expected values are shown in Figure 1. The assay was demonstrated great linearity for PINP in the range from 26.23 µg/L to 934.9 µg/L (R2=0.9987; slope=0.977; intercept=1.945) and the range from 26.23 µg/L to 934.9 µg/L (R2=0.9987; slope=0.977; intercept=1.945), respectively.
Coefficient of variances (CV) of repeatability ranged from 0.93% to 1.55%. CV of within-laboratory precision ranged from 1.11% to 2.85% (Table 2). All precision profiles for 7 samples were below manufacturer's specifications.
All results for blank samples were 5.0 µg/L for 60 replicates. Therefore, LoB was calculated as 5.0 µg/L. Results for specimens with PINP values near 5 µg/L ranged from 5.29 µg/L to 5.64 µg/L. The final LoD value of samples was determined to be 5.34 µg/L.
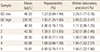
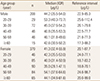
A total of 21 subjects (5 males and 16 females) were excluded after being eliminated as outliers by Tukey's test. The median and reference intervals of PINP are summarized in Table 2. In males and females, the median serum PINP levels were 44.2 µg/L (IQR, 35.5–54.0) and 41.3 µg/L (IQR, 32.4–55.9), respectively. The median PINP level in male population was slightly higher than that in females. However, such difference was not statistically significant (P=0.129). When median PINP levels were compared among age groups, the highest PINP levels in males and females were in the 20–29 age group (P=0.023) and the 50 to 59 age group (P<0.001), respectively (Table 3).
After decrement of PINP level from 20 to 29 age group to the 30 to 39 age group, PINP level remained stable in males. Decreasing tendency of PINP level in females was also found from 20 to 29 age group to 40 to 49 age group. However, PINP levels were increased sharply in the 50 to 59 age group of females (Fig. 2).
In the past decade, several BTMs have been developed to monitor bone metabolism. PINP, one of the most important BTMs, has been used to predict the bone formation.[17] In addition, reduction of PINP level has been demonstrated as a marker for predicting bone fracture in many studies.[1819] Before the development of automated assay for PINP, manual assay was the only method for measuring PINP concentrations, thus limiting the clinical application of PINP.
Recently, high-throughput automated assays for PINP have been introduced to clinical laboratories.[20] These assays could meet the increasing needs of PINP measurement with short turn-around-time and improved performance compared to manual method.
In this study, we independently evaluated the performance of automated total PINP assay on an E170 module immunology analyzer and demonstrated that the assay had excellent linearity and imprecision with very low detection limit. Especially, total PINP assay showed near perfect linearity in the range between very low concentration and extremely high concentration. In addition, repeatability and within-laboratory precision of the assay were lower than 2% and 3%, respectively. This characteristic was superior to any other PINP assays commercially available.[1321] Our data on detection capability test also suggested that extremely low concentration of PINP could be confidently reported during clinical application of this assay.
We also found negative correlation between serum PINP level and age in Korean male population. PINP level was decreased with increasing age until 40 to 49 years age group. It reached its lowest levels in the age group of 50 to 59 years. Then, there was a slightly increase from 50 to 59 age group to age group of 60+ years. Such age-related U-shape relationship has also been found in other male cohort studies.[1222] This finding might reflect active bone turnover during young age. The results of a study of Hu et al.[22] is very similar to our results of age-related reference intervals and median PINP levels in males. However, when compared to age-related reference intervals of Australian male cohort, [12] PINP levels in Korean males were lower in the age group of 20 to 29 years but higher in other age groups.
In Korean female population, negative correlation was also found between 20 to 29 and 40 to 49 years age groups. After a sharp increase in the age group of 50 to 59 years, PINP levels were decreased with increasing age. This unique pattern of PINP has been reported in populations of other countries.[121322] This might reflect the acceleration of bone turnover in post-menopausal women included in the age group of 50 to 59 years.[23]
Comparing median PINP levels between populations, Korean women showed higher levels of PINP than Australian, Belgium, or United Kingdom female populations.[121324] However, they had almost the same levels as those in a Shanghai female cohort.[22] Such results suggest that PINP levels might be different among the races (Table 4). Therefore, it is essential to establish reference intervals for each country when adopting PINP measurements for clinical applications.
Several limitations of our study should be considered. First, we did not take other hormonal markers into account, although we confirmed the normality of bone status with BMD data. Second, due to limited sample size, we had to establish reference interval in 10-year-old unit. Since we could not enroll enough number of old-aged men and women, the reference range for more than 60-year-old subjects could not be specified. Third, menopausal status was not considered. In this study, we found increment of PINP levels in female age group of 50 to 59 years. However, we could not conclude that this increase is originated from post-menopausal women due to estrogen deficiency.[5] Fourth, this study was not analyzed by BTM according to the presence of menopause.
Nevertheless, these results could contribute to the appropriate assessment of bone turnover status in Korean population. It also provides PINP levels in Korean population for comparison studies with other populations in the future.
Figures and Tables
 | Fig. 1Linearity curve of measured values of procollagen type I N-terminal propeptide compared to expected values. |
 | Fig. 2Age-related distribution of P1NP values in healthy Korean males (A) and females (B). Boxes represent inter-quartile range (bold bar, median). Whiskers extend to 5th and 95th percentile. P1NP, procollagen type I N-terminal propeptide. |
Table 2
Precision profiles of procollagen type I N-terminal propeptide measurement using quality control samples and human sera
References
1. Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994; 4:368–381.

3. Park CH, Lee YK, Ha YC. Change of bone mineral density measurement among patients with osteoporotic fractures in Korean population using national claim database. J Bone Metab. 2017; 24:183–186.

4. Yoon BH, Yu W. Clinical utility of biochemical marker of bone turnover: fracture risk prediction and bone healing. J Bone Metab. 2018; 25:73–78.

5. Hoshino H, Kushida K, Takahashi M, et al. Changes in levels of biochemical markers and ultrasound indices of Os calcis across the menopausal transition. Osteoporos Int. 2000; 11:128–133.

6. Garnero P, Hausherr E, Chapuy MC, et al. Markers of bone resorption predict hip fracture in elderly women: the EPIDOS Prospective Study. J Bone Miner Res. 1996; 11:1531–1538.

7. Emami A, Larsson A, Petrén-Mallmin M, et al. Serum bone markers after intramedullary fixed tibial fractures. Clin Orthop Relat Res. 1999; (368):220–229.

8. Orum O, Hansen M, Jensen CH, et al. Procollagen type I N-terminal propeptide (PINP) as an indicator of type I collagen metabolism: ELISA development, reference interval, and hypovitaminosis D induced hyperparathyroidism. Bone. 1996; 19:157–163.

9. Vasikaran S, Cooper C, Eastell R, et al. International osteoporosis foundation and international federation of clinical chemistry and laboratory medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011; 49:1271–1274.

10. Ivaska KK, Gerdhem P, Väänänen HK, et al. Bone turnover markers and prediction of fracture: a prospective follow-up study of 1040 elderly women for a mean of 9 years. J Bone Miner Res. 2010; 25:393–403.

11. Ardawi MS, Maimani AA, Bahksh TA, et al. Reference intervals of biochemical bone turnover markers for Saudi Arabian women: a cross-sectional study. Bone. 2010; 47:804–814.

12. Jenkins N, Black M, Paul E, et al. Age-related reference intervals for bone turnover markers from an Australian reference population. Bone. 2013; 55:271–276.

13. Morovat A, Catchpole A, Meurisse A, et al. IDS iSYS automated intact procollagen-1-N-terminus pro-peptide assay: method evaluation and reference intervals in adults and children. Clin Chem Lab Med. 2013; 51:2009–2018.

14. Brandt J, Krogh TN, Jensen CH, et al. Thermal instability of the trimeric structure of the N-terminal propeptide of human procollagen type I in relation to assay technology. Clin Chem. 1999; 45:47–53.

15. Clinical and Laboratory Standards Institute. Evaluation of precision of quantitative measurement procedures: Approved guideline. 3rd ed. Wayne, PA: CLSI document EP5-A3;2014.
16. Clinical and Laboratory Standards Institute. Evaluation of detection capability for clinical laboratory measurement procedures: Approved guideline. 2nd ed. Wayne, PA: CLSI document EP17-A2;2012.
17. Bauer DC, Sklarin PM, Stone KL, et al. Biochemical markers of bone turnover and prediction of hip bone loss in older women: the study of osteoporotic fractures. J Bone Miner Res. 1999; 14:1404–1410.

18. Bauer DC, Garnero P, Harrison SL, et al. Biochemical markers of bone turnover, hip bone loss, and fracture in older men: the MrOS study. J Bone Miner Res. 2009; 24:2032–2038.

19. Finnes TE, Lofthus CM, Meyer HE, et al. Procollagen type 1 amino-terminal propeptide (P1NP) and risk of hip fractures in elderly Norwegian men and women. A NOREPOS study. Bone. 2014; 64:1–7.

20. Garnero P, Vergnaud P, Hoyle N. Evaluation of a fully automated serum assay for total N-terminal propeptide of type I collagen in postmenopausal osteoporosis. Clin Chem. 2008; 54:188–196.

21. Koivula MK, Richardson J, Leino A, et al. Validation of an automated intact N-terminal propeptide of type I procollagen (PINP) assay. Clin Biochem. 2010; 43:1453–1457.

22. Hu WW, Zhang Z, He JW, et al. Establishing reference intervals for bone turnover markers in the healthy Shanghai population and the relationship with bone mineral density in postmenopausal women. Int J Endocrinol. 2013; 2013:513925.

23. Martínez J, Olmos JM, Hernández JL, et al. Bone turnover markers in Spanish postmenopausal women: the Camargo cohort study. Clin Chim Acta. 2009; 409:70–74.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download