Abstract
Purpose
To evaluate changes in the peripapillary retinal nerve fiber layer (RNFL) thicknesses using spectral-domain optical coherence tomography (SD-OCT) in hydroxychloroquine (HCQ) users.
Methods
The medical records of HCQ users were retrospectively reviewed. In these HCQ users, an automated perimetry, fundus autofluorescence photography, and SD-OCT with peripapillary RNFL thickness measurements were performed. The peripapillary RNFL thicknesses were compared between the HCQ users and the control groups. The relationships between the RNFL thicknesses and the duration or cumulative dosage of HCQ use were analyzed.
Results
This study included 77 HCQ users and 20 normal controls. The mean duration of HCQ usage was 63.6 ± 38.4 months, and the cumulative dose of HCQ was 528.1 ± 3.44 g. Six patients developed HCQ retinopathy. Global and six sectoral RNFL thicknesses of the HCQ users did not significantly decrease compared to those of the normal controls. No significant correlation was found between the RNFL thickness and the duration of use or cumulative dose. The eyes of those with HCQ retinopathy had temporal peripapillary RNFL thicknesses significantly greater than that of normal controls.
Hydroxychloroquine (HCQ) is a commonly used drug for the treatment of rheumatologic diseases, including systemic lupus erythematosus and rheumatoid arthritis. Retinal toxicity is a well-known side effect and can result in irreversible loss of visual acuity, central visual field defects, or decreased color vision [12]. Recent large group studies have shown that the frequency of HCQ retinopathy is much higher than previously thought [345]. At recommended doses, the risk of toxicity up to 5 years is under 1%, and the risk of toxicity up to 10 years is under 2%, but the risk rises to almost 20% after 20 years [34]. Moreover, HCQ retinopathy can be progressive even after discontinuation of the drug [6]. A prior study showed a steady progression of retinopathy for at least 3 years after HCQ is stopped when HCQ retinopathy involves retinal pigment epithelium [7].
Therefore, early detection of fundus changes is crucial for timely discontinuation of the drug. From experimental studies, early pathologic changes in patients with HCQ-related retinal toxicity were observed in the ganglion cells [89]. Accordingly, retinal ganglion cells, ganglion cell layer thicknesses, and connected retinal nerve fiber layer (RNFL) thicknesses have become the topic of investigation. In a study on patients with long-term chloroquine treatments, the RNFL thicknesses were significantly lower than in in healthy subjects, with a correlation to daily dosage [10]. In a study on patients with rheumatoid arthritis, the thinning of RNFL detected by scanning laser polarimetry (SLP) was suggested to be a useful sign of early chloroquine retinopathy [11]. In addition, peripapillary RNFL thinning on optical coherence tomography (OCT) was observed in HCQ or chloroquine users with evident retinopathy [12]. In the same study, patients without HCQ retinopathy did not show RNFL thinning. However, the exact effects of HCQ on RNFL thicknesses need further clarification due to limitations of previous studies including small sample sizes, different subject characteristics in terms of doses and the duration of HCQ use, as well as differences in measuring devices.
Currently, spectral-domain (SD)-OCT provides high resolution and high-speed image acquisition, and is considered to be a reliable method for RNFL measurements [1314151617]. In this study, we investigated whether peripapillary RNFL thicknesses changed in long-term HCQ users using SD-OCT.
The medical records of consecutive patients who received HCQ treatments for rheumatologic disease and were referred to the Department of Ophthalmology, Samsung Medical Center, for the screening of HCQ retinopathy between December 2012 and January 2016 were retrospectively reviewed. This study was approved by the institutional review board of the Samsung Medical Center (2015-10-052), and the work was conducted in accord with the Declaration of Helsinki. The informed consent was waived due to the retrospective nature of the study.
The normal controls were outpatients who required regular ocular check-ups and had no diagnosed ocular disease. The exclusion criteria were optic nerve diseases such as glaucoma, retinal diseases that could affect the peripapillary retinal structures on the SD-OCT, high myopia with spherical equivalent refractive errors more than −6.0 diopters (D), inflammatory eye diseases, and media opacity that interfered with a clear fundus or OCT examination. Subjects with an intraocular pressure of more than 21 mmHg and signs of glaucomatous optic neuropathy, such as glaucomatous visual field defects, optic disc rim thinning, notching, RNFL defects, or disc hemorrhages were excluded.
Patients that used HCQ underwent screening examinations according to the revised American Academy of Ophthalmology guidelines regarding screening for HCQ retinopathy [6]. Examinations and data collection were performed as previously described by Lee et al. [18]. In summary, dilated fundus examinations, a static automated threshold perimetry (10-2 Humphrey Field Analyzer, Model 750I; Humphrey Instruments Inc., San Leandro, CA, USA), fundus autofluorescence photography, and SD-OCT (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany) were performed. In addition, peripapillary SD-OCT was performed, and the peripapillary RNFL thickness was measured around the disc, with 47 averaged consecutive circular B-scans (diameter of 3.7 mm, 768 A-scans). An online tracking system was used to compensate for eye movements, and the RNFL thickness (from internal limiting membrane inner margin to RNFL outer margin) was automatically segmented using Spectralis software ver. 6.0.9.0. The RNFL Spectralis protocol generates a map with global (average) thicknesses, and the thicknesses of six sectors: superonasal, nasal, inferonasal, inferotemporal, temporal, and superotemporal. Images were each reviewed for segmentation acceptability. For images with segmentation errors, such as the disruption of the detected border or border wandering, manual re-segmentation was performed. Eyes with severe peripapillary atrophy that would interfere with manual adjustments of segmentation were excluded.
HCQ users were grouped into three subgroups: group A, patients with HCQ use over 5 years; group B, patients with HCQ retinopathy; and group C, patients without HCQ retinopathy. The right eye of each patient was analyzed. Global RNFL thicknesses and six sectoral RNFL thicknesses were compared between all HCQ users and controls, and between subgroups A, B, and C and the controls. A Pearson correlation analysis was conducted between RNFL thicknesses, the duration of HCG use, and the cumulative dose of HCQ. All statistical analysis of data was carried out using IBM SPSS Statistics ver. 22 (IBM Corp., Armonk, NY, USA), and the significance level was set at a p < 0.05.
Finally, 77 HCQ users and 20 normal controls were analyzed. Demographic and clinical characteristics of all 97 subjects are shown in Table 1. The two groups showed no statistically significant differences in age and refractive errors, which are known to be associated with RNFL thickness. However, the proportion of females was significantly higher in the HCQ group than in the controls (p < 0.001). There was no sign of concomitant glaucomatous optic nerve damage in the HCQ users or in the normal controls.
In the HCQ users, the mean duration of HCQ use was 63.6 months, and the mean total exposure to HCQ was 528.1 g. The mean daily dosage was 291.4 mg, and the mean daily dosage per kilogram of ideal body weight was 5.64 mg/kg. The clinical characteristics of all HCQ users and the 3 subgroups are presented in Table 2. Among the 77 patients with HCQ therapy, 6 patients (7.8%) were diagnosed with definite or probable HCQ retinopathy. The mean duration of HCQ use in this subgroup was 118.2 months, and the mean cumulative dose was 910.5 g (Table 2).
For all HCQ users, the mean average global RNFL thickness was 101.2 µm, which was not significantly different from that of the normal controls (p = 0.645). None of the six sectoral RNFL thicknesses showed a significant difference between the HCQ users and the normal controls (all p > 0.05) (Table 3). In the subgroup analysis, the mean temporal RNFL thickness in group B was significantly higher than that of the normal controls (p = 0.039) (Table 3).
The correlation analysis showed that the RNFL thicknesses in global, nasal, and temporal areas were not significantly correlated with duration or cumulative dose of HCQ for all HCQ users (Table 4 and Fig. 1A–1F). To exclude the possible outlier effects of six patients with HCQ retinopathy who showed thicker temporal RNFL thicknesses, the correlation between the RNFL thicknesses in the HCQ users without HCQ retinopathy (group C), and the duration and cumulative doses of HCQ were analyzed. This analysis also revealed that neither the duration nor the cumulative dose significantly correlated with the RNFL thicknesses of the global, nasal, or temporal areas. Cases of HCQ retinopathy are presented in Fig. 2A–2E and 3A–3F, which showed no evidence of peripapillary RNFL thinning.
We previously demonstrated that macular ganglion cell-inner plexiform layer (GC-IPL) thinning is observed in some patients with long-term HCQ use [18]. Recently, GC-IPL thinning in patients with HCQ therapy was demonstrated using SD-OCT [19]. Additionally, several studies on a small number of subjects with limited duration of HCQ usage showed RNFL thinning in patients with exposure to HCQ [101112]. In this study, we investigated whether peripapillary RNFL thinning was present in long-term HCQ users. Contrary to previous reports, our results revealed that peripapillary RNFL thicknesses did not change in HCQ users. Peripapillary RNFL thicknesses also did not show a correlation with the duration of HCQ use or cumulative dose of HCQ.
Experimental studies suggested that in HCQ-related toxicity in the retina, early pathologic changes are observed in ganglion cells [89]. RNFL is the collection of axon bundles of ganglion cell bodies, and thus RNFL thinning is possible with ganglion cell damage. Until now, only a limited number of studies with relatively small sample sizes have been reported. The imaging modalities, sample size, and prescribed medication for rheumatologic treatment vary between studies. Results are conflicting, and conclusions remain controversial [101112]. In a study by Bonanomi et al. [10] on 34 chloroquine users, RNFL thicknesses measured by SLP decreased in HCQ users. Xiaoyun et al. [11] also reported RNFL thinning in 60 chloroquine users using a scanning laser polarimeter. However, in their study, only two of 60 patients had visual field defects, and the duration of HCQ usage (mean 42 months) was relatively short.
SLP results are largely influenced by the scan status [20] and the polarizing properties of the cornea and crystalline lens, which may lead to spurious measurements [21]. It was shown that SLP with variable corneal compensation, SLP with enhanced cornea compensation, and SD-OCT RNFL measurements cannot be directly compared [22]. It was suggested that although compensation methods improve correlation with visual function and measured RNFL thicknesses with SLP [2324], they may not be sufficiently sensitive in glaucoma [25]. When the repeatability of RNFL thickness measurements were compared between Fourier-domain OCT and SLP devices, OCT demonstrated better results [26]. OCT was also better with red-free photography in RNFL defects than in SLP [27]. Moreover, eyes with macular pathology require caution to ensure adequacy of corneal compensation [28]. Moreover, OCT-based RNFL thickness measurements are the currently generalized method in glaucoma. Together, these results indicate that measurements with OCT are more reliable than with SLP. A recent SD-OCT study in HCQ users should be emphasized in the same context. In that study, RNFL thinning was observed in patients with diagnosed HCQ retinopathy, while patients without HCQ retinopathy did not show RNFL thinning [12]. Despite the small study size (four eyes with HCQ retinopathy, eight eyes with HCQ use, and eight controls), the negative finding is consistent with our results.
Our study sample was larger than in any previous studies, and elaborate manipulation on RNFL layer segmentation was performed to eliminate any error in measurements. The possibility of erroneous examination or effects of any artifacts had to be ruled out in interpreting SD-OCT images [2930]. The control eyes were restricted to those who had no known factors for OCT measurement deviation from the normal population. Patients with high myopia, large peripapillary atrophy, and suspicious glaucomatous disc changes were strictly excluded. For every OCT scan, images were carefully examined and manually re-segmented if any segmentation failure was observed.
Previous studies suggesting peripapillary RNFL thinning in HCQ users also reported significant correlations between RNFL thicknesses and daily doses or cumulative doses. Conversely, we did not find correlations between RNFL thicknesses and cumulative doses or the duration of drug use. On the other hand, paradoxical thickening of RNFL was observed in a portion of study patients with HCQ retinopathy. Thickening of RNFL associated with retinal diseases has been reported in retinitis pigmentosa and drug-related changes. In retinitis pigmentosa, both abnormal thickening and RNFL thinning have been identified [313233]. The incidences ranged from 20% to 40% of cases. In a case report of early-stage ethambutol-induced optic neuropathy, transient thickening of peripapillary RNFL was observed. This case showed increased temporal peripapillary RNFL thickness and simultaneous decreased perifoveal GC-IPL thickness. The authors stated that RNFL swelling in the temporal sector had been observed in other optic neuropathies, including early-stage linezolid-associated optic neuropathy and Leber's hereditary optic neuropathy [3435]. The exact mechanism and clinical implications of increased temporal peripapillary RNFL thickness in HCQ users are uncertain. For causes of RNFL thickening observed in a limited number of severely affected HCQ retinopathy patients, we considered glial proliferation, remodeling including glial hypertrophy, or axonal swelling as possible candidates, as they were suspected to be causative in RP patients [3637]. Thickening was not transient, no peripapillary hemorrhage was observed, and they not limited to the peripapillary areas, but extended further into the retina, suggesting the unlikeliness of papilledema associated with inflammation. Rather, chronic reactive change secondary to RGC soma and axonal degeneration would better explain the thickening of RNFL. Moreover, as it seems that severity of disease might affect the presence of pathologic RNFL thickening, we consider glial cell reaction associated with neural loss as a likely answer for the phenomenon. Further studies with a larger number of patients with HCQ retinopathy are warranted.
Our study had several limitations. First, the retrospective design and small number of patients with HCQ retinopathy made it difficult to draw definite conclusions regarding the clinical significance of RNFL thickness in HCQ users. The incidence of HCQ retinopathy was relatively low, and a study of a large number of patients with HCQ retinopathy was not feasible. Nevertheless, this study included the largest number of HCQ patients in the literature among studies on changes in RNFL thickness. Second, the control group consisted of healthy normal adults, but an ideal group for comparison would have been patients diagnosed with a similar group of rheumatologic diseases without HCQ use. Because there are few opportunities to perform ocular examinations on patients with rheumatologic diseases without reason, recruitment of such a control group would be challenging. Future studies with adequate rheumatologic disease controls would provide better information for HCQ users. Third, because this was a cross-sectional study, longitudinal changes in patients with HCQ retinopathy or long-term users could not be investigated. Further studies on RNFL thickness changes over longer periods of time are warranted.
Peripapillary RNFL thicknesses did not change in HCQ users, and did not show correlations with the duration of HCQ use or with cumulative doses of HCQ. Therefore, RNFL thicknesses are not a useful biomarker for the early detection of HCQ retinal toxicity. Detailed characteristics and clinical implications of temporal RNFL thickening observed in HCQ retinopathy need further investigation.
Figures and Tables
 | Fig. 1Peripapillary retinal nerve fiber layer (RNFL) thicknesses in hydroxychloroquine (HCQ) users and normal controls. The relationship between RNFL thickness, duration of use, and cumulative doses are shown together. The distribution of global RNFL thicknesses is not significantly different from the normal controls in all HCQ users, and there was no definite relationship between cumulative doses (A) or duration of use (B). The result was consistent in the temporal (C,D) and nasal (E,F) sectors. Note: some eyes with HCQ retinopathy showed high RNFL thickness values. Red dots indicated patients with HCQ retinopathy, green dots indicated patients with HCQ users over 5 years and without HCQ retinopathy, and yellow dots indicated normal controls. |
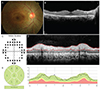 | Fig. 2Representative images of the right eye of a 33-year-old female diagnosed with rheumatoid arthritis. This patient had chronic exposure to a total of 786 g of hydroxychloroquine for 119 months. Her daily dosage per ideal body weight was 4.43 mg/kg. The best-corrected visual acuity was 20 / 20 in both eyes. The color fundus photograph (A) and the optical coherence tomography (B) shows severe hydroxychloroquine retinopathy with outer retinal collapse in the peripheral fovea. (C) Humphrey 10-2 visual field testing shows ring scotoma. (D) Despite collapsed outer retinal layer, retinal nerve fiber layer thickening was observed in the circular B-scan. (E) Spectralis spectral-domain optical coherence tomography measurements of the peripapillary retinal nerve fiber layer thickness showed no area of thinning. |
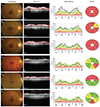 | Fig. 3Six hydroxychloroquine retinopathy patients and their peripapillary retinal nerve fiber layer (RNFL) thickness profiles and ganglion cell-inner plexiform layer thicknesses. (A–C) Three patients with RNFL thickening showed more severe ganglion cell damage than (D–F) other three patients without RNFL thickening. OCT = optical coherence tomography; GC-IPL = ganglion cell-inner plexiform layer; TMP = temporal; SUP = superior; NAS = nasal; INF = inferior. |
Table 1
Demographic and clinical characteristics of the hydroxychloroquine users and the controls

Values are presented as mean ± standard deviation or number (%).
NA = not applicable.
*p-value by chi-square test for categorical variables and independent t-test for continuous variables; †Five patients were diagnosed with more than one disease; two patients were diagnosed with antiphospholipid antibody syndrome, two patients were diagnosed with Sjögren syndrome, and one patient was diagnosed with dermatomyositis; ‡Two patients were diagnosed with antiphospholipid antibody syndrome, and one patient was diagnosed with mixed connective tissue disease.
Acknowledgements
This work was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1826).
References
1. Bernstein HN. Chloroquine ocular toxicity. Surv Ophthalmol. 1967; 12:415–447.
2. Mavrikakis I, Sfikakis PP, Mavrikakis E, et al. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003; 110:1321–1326.
3. Marmor MF, Kellner U, Lai TY, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016; 123:1386–1394.

4. Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014; 132:1453–1460.

5. Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015; 122:110–116.

6. Marmor MF, Kellner U, Lai TY, et al. Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011; 118:415–422.

7. Marmor MF, Hu J. Effect of disease stage on progression of hydroxychloroquine retinopathy. JAMA Ophthalmol. 2014; 132:1105–1112.

8. Hallberg A, Naeser P, Andersson A. Effects of long-term chloroquine exposure on the phospholipid metabolism in retina and pigment epithelium of the mouse. Acta Ophthalmol (Copenh). 1990; 68:125–130.

9. Rosenthal AR, Kolb H, Bergsma D, et al. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978; 17:1158–1175.
10. Bonanomi MT, Dantas NC, Medeiros FA. Retinal nerve fibre layer thickness measurements in patients using chloroquine. Clin Exp Ophthalmol. 2006; 34:130–136.

11. Xiaoyun MA, Dongyi HE, Linping HE. Assessing chloroquine toxicity in RA patients using retinal nerve fibre layer thickness, multifocal electroretinography and visual field test. Br J Ophthalmol. 2010; 94:1632–1636.

12. Pasadhika S, Fishman GA. Effects of chronic exposure to hydroxychloroquine or chloroquine on inner retinal structures. Eye (Lond). 2010; 24:340–346.

13. Tan BB, Natividad M, Chua KC, Yip LW. Comparison of retinal nerve fiber layer measurement between 2 spectral domain OCT instruments. J Glaucoma. 2012; 21:266–273.

14. Asrani S, Essaid L, Alder BD, Santiago-Turla C. Artifacts in spectral-domain optical coherence tomography measurements in glaucoma. JAMA Ophthalmol. 2014; 132:396–402.

15. Wheat JL, Rangaswamy NV, Harwerth RS. Correlating RNFL thickness by OCT with perimetric sensitivity in glaucoma patients. J Glaucoma. 2012; 21:95–101.

16. Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006; 59:963–969.

17. Ratchford JN, Quigg ME, Conger A, et al. Optical coherence tomography helps differentiate neuromyelitis optica and MS optic neuropathies. Neurology. 2009; 73:302–308.

18. Lee MG, Kim SJ, Ham DI, et al. Macular retinal ganglion cell-inner plexiform layer thickness in patients on hydroxychloroquine therapy. Invest Ophthalmol Vis Sci. 2014; 56:396–402.

19. Uslu H, Gurler B, Yildirim A, et al. Effect of hydroxychloroquine on the retinal layers: a quantitative evaluation with spectral-domain optical coherence tomography. J Ophthalmol. 2016; 2016:8643174.

20. Hoesl LM, Tornow RP, Schrems WA, et al. Glaucoma diagnostic performance of GDxVCC and spectralis OCT on eyes with atypical retardation pattern. J Glaucoma. 2013; 22:317–324.

21. Lemij HG. The value of polarimetry in the evaluation of the optic nerve in glaucoma. Curr Opin Ophthalmol. 2001; 12:138–142.

22. Schallenberg M, Dekowski D, Kremmer S, et al. Comparison of Spectralis-OCT, GDxVCC and GDxECC in assessing retinal nerve fiber layer (RNFL) in glaucomatous patients. Graefes Arch Clin Exp Ophthalmol. 2013; 251:1343–1353.

23. Bagga H, Greenfield DS, Feuer W, Knighton RW. Scanning laser polarimetry with variable corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Am J Ophthalmol. 2003; 135:521–529.

24. Sehi M, Ume S, Greenfield DS. Scanning laser polarimetry with enhanced corneal compensation and optical coherence tomography in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2007; 48:2099–2104.

25. Kook MS, Cho HS, Seong M, Choi J. Scanning laser polarimetry using variable corneal compensation in the detection of glaucoma with localized visual field defects. Ophthalmology. 2005; 112:1970–1978.

26. Garas A, Toth M, Vargha P, Hollo G. Comparison of repeatability of retinal nerve fiber layer thickness measurement made using the RTVue Fourier-domain optical coherence tomograph and the GDx scanning laser polarimeter with variable or enhanced corneal compensation. J Glaucoma. 2010; 19:412–417.

27. Oh JH, Kim YY. Scanning laser polarimetry and optical coherence tomography for detection of retinal nerve fiber layer defects. Korean J Ophthalmol. 2009; 23:169–175.

28. Bagga H, Greenfield DS, Knighton RW. Scanning laser polarimetry with variable corneal compensation: identification and correction for corneal birefringence in eyes with macular disease. Invest Ophthalmol Vis Sci. 2003; 44:1969–1976.

29. Giani A, Cigada M, Esmaili DD, et al. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina. 2010; 30:607–616.

30. Sull AC, Vuong LN, Price LL, et al. Comparison of spectral/Fourier domain optical coherence tomography instruments for assessment of normal macular thickness. Retina. 2010; 30:235–245.

31. Anastasakis A, Genead MA, McAnany JJ, Fishman GA. Evaluation of retinal nerve fiber layer thickness in patients with retinitis pigmentosa using spectral-domain optical coherence tomography. Retina. 2012; 32:358–363.

32. Hwang YH, Kim SW, Kim YY, et al. Optic nerve head, retinal nerve fiber layer, and macular thickness measurements in young patients with retinitis pigmentosa. Curr Eye Res. 2012; 37:914–920.

33. Walia S, Fishman GA. Retinal nerve fiber layer analysis in RP patients using Fourier-domain OCT. Invest Ophthalmol Vis Sci. 2008; 49:3525–3528.

34. Han J, Lee K, Rhiu S, et al. Linezolid-associated optic neuropathy in a patient with drug-resistant tuberculosis. J Neuroophthalmol. 2013; 33:316–318.

35. Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber's hereditary optic neuropathy: longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010; 117:623–627.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download