초록
Purpose
This study was done to identify the changes of cognitive function and depression following Chemotherapy in women with breast cancer.
Methods
Fifty patients participated in the study and completed the questionnaire at three-time points: pre-chemotherapy, post-chemotherapy, and six months after the completion of chemotherapy. The assessment tools were: everyday cognition, the Montreal Cognitive Assessment, and the Hospital Anxiety and Depression Scale. Data were analyzed using descriptive statistics and repeated measures analysis of variance.
Results
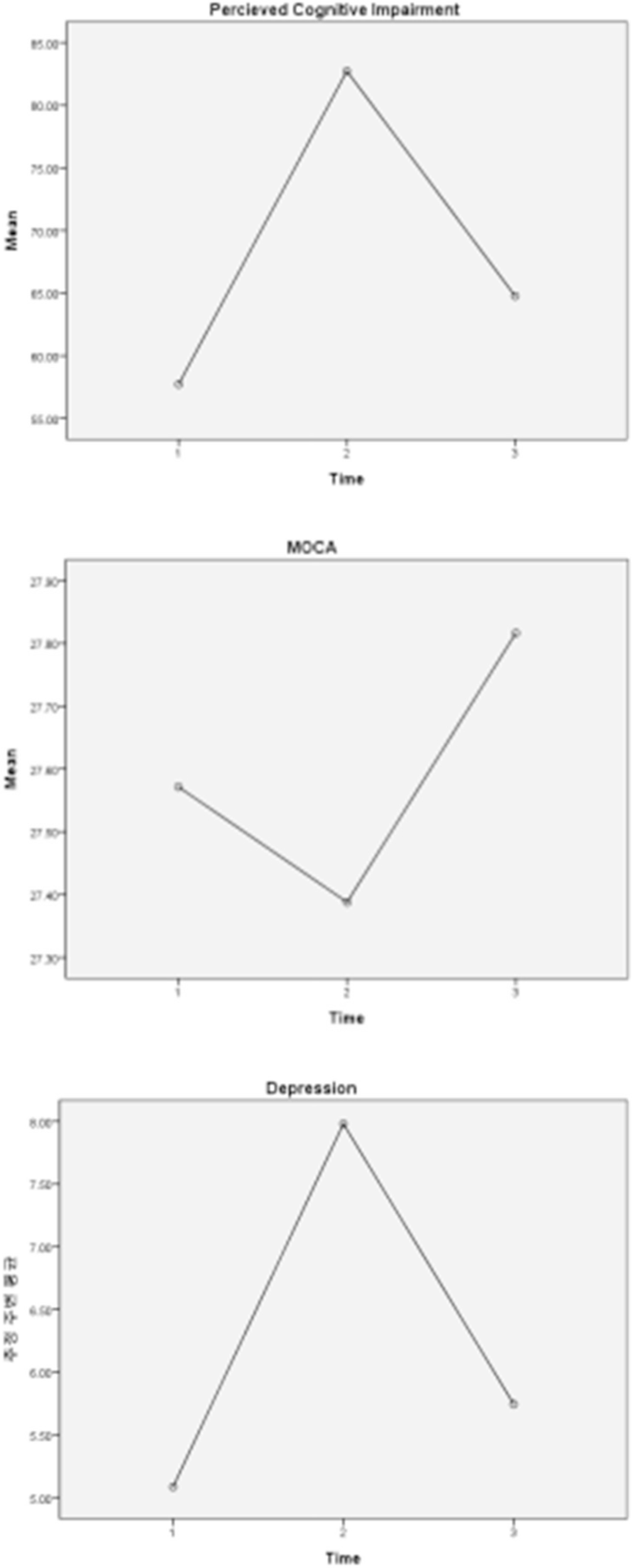
Immediately after chemotherapy, 52.0% of patients complained of subjective cognitive decline and reported greater difficulty in the cognitive domains of attention, memory, and visuospatial abilities. At six-month Follow-up, 24.0% of patients exhibited mild cognitive decline. Repeated measures ANOVA showed a significant decline in cognitive function after chemotherapy. However, improvement was observed 6 months after the completion of chemotherapy. Depression showed similar patterns to cognitive function. Higher cognitive decline scores were significantly correlated with higher depression (r=.33, p=.020).
Go to : 
REFERENCES
1. Kim SH, Lee R, Lee KS. Symptom clusters in patients with breast cancer. J Korean Acad Adult Nurs. 2009; 21:705–17.
2. Calvio L, Peugeot M, Bruns GL, Todd BL, Feuerstein M. Measures of cognitive function and work in occupationally active breast cancer survivors. J Occup Environ Med. 2010; 52:219–27.

3. Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009; 18:134–43.

4. Hutchinson AD, Hosking JR, Kichenadasse G, Mattiske JK, Wilson C. Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev. 2012; 38:926–34.

5. de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, Caan M, Douaud G, et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: converging results from multi-modal magnetic resonance imaging. Hum Brain Mapp. 2012; 33:2971–83.

6. Lange M, Rigal O, Clarisse B, Giffard B, Sevin E, Barillet M, et al. Cognitive dysfunctions in elderly cancer patients: a new challenge for on-cologists. Cancer Treat Rev. 2014; 40:810–7.

7. Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuro-psychological assessment. 4th ed.New York, NY: Oxford University Press;2004.
8. Kang MA, Baek YM. The neurocognitive function between the patients who had subjective memory impairment and mild cognitive impairment. J Korean Geriatr Soc. 2014; 18:7–15.

9. Hyrien O, Dietrich J, Noble M. Mathematical and experimental approaches to identify and predict the effects of chemotherapy on neuro-glial precursors. Cancer Res. 2010; 70:10051–9.

10. Nelson CJ, Nandy N, Roth AJ. Chemotherapy and cognitive deficits: mechanisms, findings, and potential interventions. Palliat Support Care. 2007; 5:273–80.

11. Park JH, Bae SH, Jung YS, Jung YM. Prevalence and characteristics of chemotherapy-related cognitive impairment in patients with breast cancer. J Korean Acad Nurs. 2015; 45:118–28.

12. Chung BY, Cho EJ. Correlates influencing cognitive impairment in breast cancer patients receiving chemotherapy. Asian Oncol Nurs. 2012; 12:221–9.

13. Kaiser J, Bledowski C, Dietrich J. Neural correlates of chemotherapy-related cognitive impairment. Cortex. 2014; 54:33–50.

14. Ashbury FD, Madlensky L, Raich P, Thompson M, Whitney G, Hotz K, et al. Antidepressant prescribing in community cancer care. Support Care Cancer. 2003; 11:278–85.

15. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53:695–9.

16. Lee JY, Lee DW, Cho SJ, Na DL, Jeon HJ, Kim SK, et al. Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2008; 21:104–10.

17. Oh SM, Min KJ, Park DB. A study on the standardization of the hospital anxiety and depression scale for Koreans: a comparison of normal, depressed and anxious groups. J Korean Neuropsychiatr Assoc. 1999; 38:289–96.
18. Chaiy SI. Soical science research methodology. 2nd ed.Seoul: Hakhy-unsa;2000.
19. Jansen CE, Cooper BA, Dodd MJ, Miaskowski CA. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011; 19:1647–56.

20. Collins B, Mackenzie J, Kyeremanteng C. Study of the cognitive effects of chemotherapy: considerations in selection of a control group. J Clin Exp Neuropsychol. 2013; 35:435–44.

21. Lindner OC, Phillips B, McCabe MG, Mayes A, Wearden A, Varese F, et al. A metaanalysis of cognitive impairment following adult cancer chemotherapy. Neuropsychology. 2014; 28:726–40.

22. Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. Eur J Oncol Nurs. 2013; 17:236–41.

23. Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013; 39:297–304.

24. Myers JS. Chemotherapy-related cognitive impairment: the breast cancer experience. Oncol Nurs Forum. 2012; 39:E31–40.

25. Lee JR, Oh PJ. Cognitive decline and quality of life among patients with breast cancer undergoing chemotherapy: the mediating effect of health promotion behavior. Korean J Adult Nurs. 2016; 28:202–12.

26. Henderson VW. Cognitive changes after menopause: influence of es-trogen. Clin Obstet Gynecol. 2008; 51:618–26.

27. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mecha-nisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014; 26:102–13.

28. Oh PJ, Lee JR. Effect of cancer symptoms and fatigue on chemotherapy-related cognitive impairment and depression in people with gastrointestinal cancer. J Korean Acad Nurs. 2016; 46:420–30.

29. Lee JR. A structural model for chemotherapy related cognitive change in breast cancer patients [dissertation]. Seoul: Sahmyook Univ.;2016.
Go to : 
Table 1.
Cognitive Function and Depression according to General Characteristics of the Subject (N = 50)
Table 2.
Correlation among the Perceived Cognitive Decline, Objective Cognitive Function and Depression (N =50)
Table 3.
Changes in Number of Participants Experiencing Cognitive Decline and Depression from Baseline to Follow-up
Table 4.
Changes in Cognitive Function and Depression from Baseline to Follow-up (N =47)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download