1. Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician's Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008; 11:473–477. PMID:
18562228.

2. Cummings SR, Kelsey JL, Nevitt MC, O'Dowd KJ. Epidemiology of osteoporosis and osteoporotic fractures. Epidemiol Rev. 1985; 7:178–208. PMID:
3902494.

3. Cooper C, Atkinson EJ, Jacobsen SJ, O'Fallon WM, Melton LJ 3rd. Population-based study of survival after osteoporotic fractures. Am J Epidemiol. 1993; 137:1001–1005. PMID:
8317445.

4. Lyles KW, Colón-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007; 357:1799–1809. PMID:
17878149.

5. Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000; 15:721–739. PMID:
10780864.

6. Schürch MA, Rizzoli R, Mermillod B, Vasey H, Michel JP, Bonjour JP. A prospective study on socioeconomic aspects of fracture of the proximal femur. J Bone Miner Res. 1996; 11:1935–1942. PMID:
8970896.

7. Trombetti A, Herrmann F, Hoffmeyer P, Schurch MA, Bonjour JP, Rizzoli R. Survival and potential years of life lost after hip fracture in men and age-matched women. Osteoporos Int. 2002; 13:731–737. PMID:
12195537.

8. Wolinsky FD, Fitzgerald JF, Stump TE. The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. Am J Public Health. 1997; 87:398–403. PMID:
9096540.

9. Andrade SE, Majumdar SR, Chan KA, et al. Low frequency of treatment of osteoporosis among postmenopausal women following a fracture. Arch Intern Med. 2003; 163:2052–2057. PMID:
14504118.

10. Gong HS, Oh WS, Chung MS, Oh JH, Lee YH, Baek GH. Patients with wrist fractures are less likely to be evaluated and managed for osteoporosis. J Bone Joint Surg Am. 2009; 91:2376–2380. PMID:
19797572.

11. Giangregorio L, Papaioannou A, Cranney A, Zytaruk N, Adachi JD. Fragility fractures and the osteoporosis care gap: an international phenomenon. Semin Arthritis Rheum. 2006; 35:293–305. PMID:
16616152.

12. Torgerson DJ, Dolan P. Prescribing by general practitioners after an osteoporotic fracture. Ann Rheum Dis. 1998; 57:378–379. PMID:
9771215.

13. Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000; 109:326–328. PMID:
10996585.

14. Melton LJ 3rd, Chrischilles EA, Cooper C, Lane AW, Riggs BL. Perspective. How many women have osteoporosis? J Bone Miner Res. 1992; 7:1005–1010. PMID:
1414493.

15. Freedman KB, Kaplan FS, Bilker WB, Strom BL, Lowe RA. Treatment of osteoporosis: are physicians missing an opportunity? J Bone Joint Surg Am. 2000; 82-A:1063–1070. PMID:
10954094.

16. Miller PD, Zapalowski C, Kulak CA, Bilezikian JP. Bone densitometry: the best way to detect osteoporosis and to monitor therapy. J Clin Endocrinol Metab. 1999; 84:1867–1871. PMID:
10372677.

17. Borders J, Kerr E, Sartoris DJ, et al. Quantitative dual-energy radiographic absorptiometry of the lumbar spine: in vivo comparison with dual-photon absorptiometry. Radiology. 1989; 170:129–131. PMID:
2909085.

18. Sartoris DJ, Resnick D. Current and innovative methods for noninvasive bone densitometry. Radiol Clin North Am. 1990; 28:257–278. PMID:
2408094.
19. Wahner HW, Dunn WL, Brown ML, Morin RL, Riggs BL. Comparison of dual-energy x-ray absorptiometry and dual photon absorptiometry for bone mineral measurements of the lumbar spine. Mayo Clin Proc. 1988; 63:1075–1084. PMID:
3193817.

20. Crilly RG, Kloseck M, Chesworth B, Mequanint S, Sadowski E, Gilliland J. Comparison of hip fracture and osteoporosis medication prescription rates across Canadian provinces. Osteoporos Int. 2014; 25:205–210. PMID:
23907572.

21. Grover M, Anderson M, Gupta R, et al. Increased osteoporosis screening rates associated with the provision of a preventive health examination. J Am Board Fam Med. 2009; 22:655–662. PMID:
19897694.

22. Kalender WA. Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int. 1992; 2:82–87. PMID:
1536984.

23. Huda W, Morin RL. Patient doses in bone mineral densitometry. Br J Radiol. 1996; 69:422–425. PMID:
8705180.

24. Kalender WA, Buchenau S, Deak P, et al. Technical approaches to the optimisation of CT. Phys Med. 2008; 24:71–79. PMID:
18331808.

25. Damilakis J, Adams JE, Guglielmi G, Link TM. Radiation exposure in X-ray-based imaging techniques used in osteoporosis. Eur Radiol. 2010; 20:2707–2714. PMID:
20559834.

26. Autier P, Haentjens P, Bentin J, et al. Belgian Hip Fracture Study Group. Costs induced by hip fractures: a prospective controlled study in Belgium. Osteoporos Int. 2000; 11:373–380. PMID:
10912837.

27. Haentjens P, Autier P, Barette M, Boonen S. Belgian Hip Fracture Study Group. The economic cost of hip fractures among elderly women. A one-year, prospective, observational cohort study with matched-pair analysis. J Bone Joint Surg Am. 2001; 83-A:493–500. PMID:
11315777.
28. Sambrook P, Cooper C. Osteoporosis. Lancet. 2006; 367:2010–2018. PMID:
16782492.

29. Zethraeus N, Ben Sedrine W, Caulin F, et al. Models for assessing the cost-effectiveness of the treatment and prevention of osteoporosis. Osteoporos Int. 2002; 13:841–857. PMID:
12415431.

30. Boonen S, Autier P, Barette M, Vanderschueren D, Lips P, Haentjens P. Functional outcome and quality of life following hip fracture in elderly women: a prospective controlled study. Osteoporos Int. 2004; 15:87–94. PMID:
14605799.
31. Li C, Mori S, Li J, et al. Long-term effect of incadronate disodium (YM-175) on fracture healing of femoral shaft in growing rats. J Bone Miner Res. 2001; 16:429–436. PMID:
11277259.

32. Cao Y, Mori S, Mashiba T, et al. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002; 17:2237–2246. PMID:
12469918.

33. Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005; 90:1294–1301. PMID:
15598694.

34. Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures A prospective randomized intervention. J Bone Joint Surg Am. 2008; 90:953–961. PMID:
18451385.
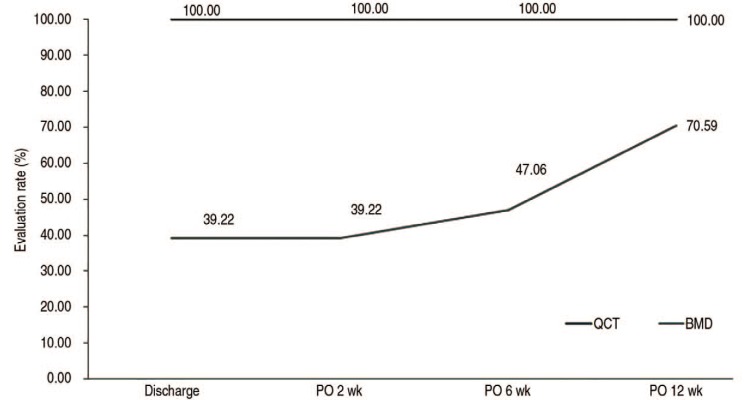
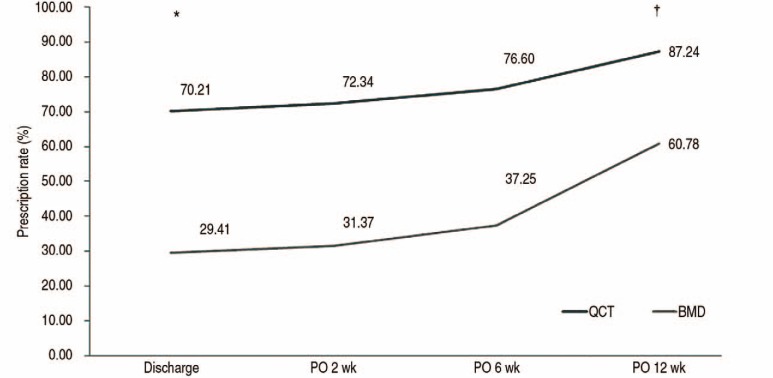

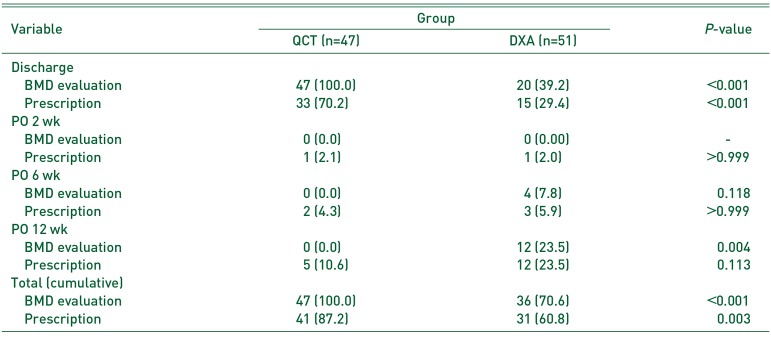




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download