Abstract
Background
Epidemiological studies have suggested an association between Hashimoto thyroiditis (HT) and papillary thyroid cancer (PTC) development. Other studies, however, have reported a protective role of HT against PTC progression. Through this updated meta-analysis, we aimed to clarify the effects of HT on the progression of PTC.
Methods
We searched citation databases, including PubMed and Embase, for relevant studies from inception to September 2017. From these studies, we calculated the pooled odds ratios (ORs) of clinicopathologic features and the relative risk (RR) of PTC recurrence with 95% confidence intervals (CIs) using the Mantel-Haenszel method. Additionally, the Higgins I2 statistic was used to test for heterogeneity.
Results
The meta-analysis included 71 published studies with 44,034 participants, among whom 11,132 had HT. We observed negative associations between PTC with comorbid HT and extrathyroidal extension (OR, 0.74; 95% CI, 0.68 to 0.81), lymph node metastasis (OR, 0.82; 95% CI, 0.72 to 0.94), distant metastasis (OR, 0.49; 95% CI, 0.32 to 0.76), and recurrence (RR, 0.50; 95% CI, 0.41 to 0.61).
Hashimoto thyroiditis (HT), also known as chronic lymphocytic thyroiditis, is the most common human autoimmune disease, with an incidence estimated to range from 0.3 to 1.5 cases per 1,000 people [12]. HT is characterized by a cellular immune response involving T and B lymphocytic infiltration of the thyroid gland, as well as by a humoral immune response leading to the production of thyroid-specific antibodies [3].
Papillary thyroid cancer (PTC) is the most common histologic type of thyroid cancer, and its incidence is increasing rapidly worldwide [4]. Notably, epidemiological studies have reported an average coexistence rate between HT and PTC of approximately 23% (range, 5% to 85%) [5]. Although the mechanism underlying this association is not fully understood, several experimental studies have suggested that the synchronous appearance of HT and PTC reflects an immunological link. Consistent with these findings, a recent meta-analysis of 76,281 patients in 27 studies observed HT in patients with PTC more frequently than in those with benign thyroid diseases [6]. Apart from its positive association with the development of PTC, interestingly, HT has also been suggested to play a protective role against the progression of PTC [5789]. A meta-analysis [5] revealed that PTC patients with coexisting HT exhibited less aggressive clinicopathologic characteristics, as manifested by lower rates of extrathyroidal extension (ETE) and lymph node metastasis, and showed a longer recurrence-free survival duration. However, that meta-analysis only included four studies and did not present a subgroup analysis according to potential confounding factors, such as tumor size.
Given the limitations of previous work and the subsequent publications in this field, an updated review and meta-analysis of recent data is warranted to better understand and clarify the effects of HT on the progression of PTC. Therefore, we performed an updated meta-analysis via a comprehensive investigation of the literature and a predefined subgroup analysis.
The literature search was performed in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Supplemental Table S1). Two independent investigators (S.M. and H.S.C.) searched the PubMed and Embase databases and selected articles using combinations of the following terms: “Hashimoto's thyroiditis” or “chronic lymphocytic thyroiditis” and “thyroid cancer.” Only articles published in English prior to September 2017 were included.
The literature search yielded 1,132 potentially relevant articles, of which 982 were screened for further review after excluding duplicate studies. If a single study was described in multiple reports, the latest or most complete publication was included. All articles were downloaded and screened for inclusion using a 2-step method. After an evaluation of the titles and abstracts according to predefined criteria, 898 articles were excluded because they met one or more of the following criteria: (1) a different topic of interest; (2) an animal or in vitro study; (3) no information about HT or thyroid cancer; or (4) publication as an abstract, expert opinion, conference article, or review. Subsequently, the full texts of the 85 remaining articles were reviewed by two independent investigators (S.M. and H.S.C.), and any disagreement was resolved by a third investigator (Y.J.P.). Seventy-two articles were finally selected for the meta-analysis (Fig. 1).
Three researchers independently assessed the methodological quality of the included articles using the Newcastle-Ottawa Scale for case-control studies [10]. Eight items were included in the quality assessment, and all articles received scores above 5 of 9. We concluded that the quality of these cross-sectional studies would not affect the quality of our meta-analysis.
The following variables were independently extracted by the two investigators using the same criteria: first author, publication year, country, number of study participants, number of cases of coexistence of HT, sex ratio, and the clinicopathologic features and recurrence of PTC.
We used the Mantel-Haenszel method to calculate the pooled odds ratios (ORs) or relative risks (RRs) with 95% confidence intervals (CIs). The Higgins I2 statistic was used to test for heterogeneity. Here, an I2 ≤50% indicated that the included studies had little heterogeneity, and a fixed-effects model was used; by contrast, an I2 >50% indicated heterogeneity, and a random-effects model was used. Subgroup and sensitivity analyses were used to determine the cause of heterogeneity. The Egger test and a funnel plot analysis were used to determine the likelihood of publication bias. All statistical analyses were performed using the statistical program R (R version 3.1.0, 2014, R Project for Statistical Computing, Vienna, Austria; www.r-project.org).
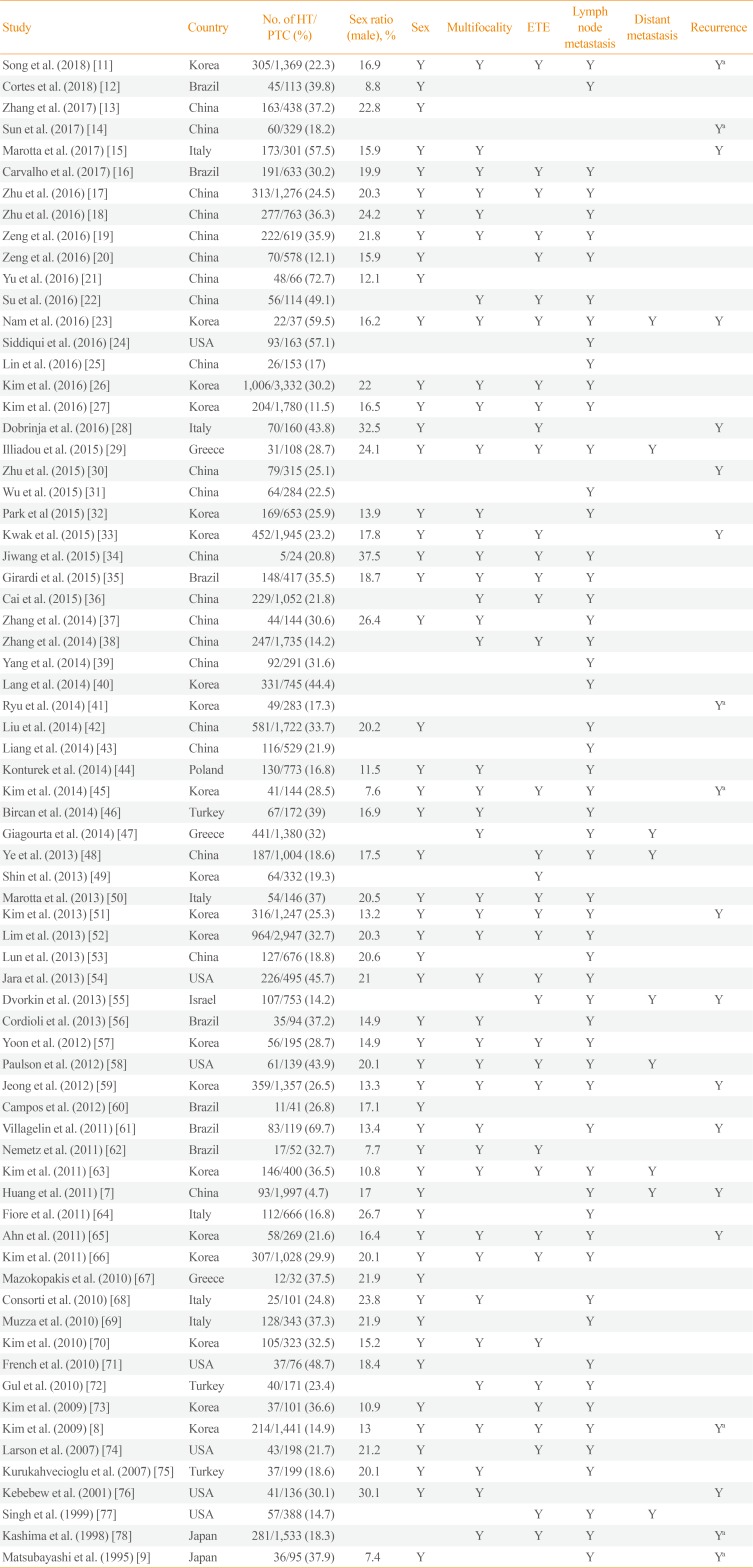
The main characteristics of the 71 articles included in this meta-analysis are summarized in Table 1 [7891112131415161718192021222324252627282930313233343536373839404142434445464748495051525354555657585960616263646566676869707172737475767778]. A total of 44,034 participants with PTC were enrolled, of whom 11,132 (25.3%) had HT. The sample sizes of these studies ranged from 24 to 3,332 participants. Among them, 53 studies reported the mean tumor size of PTC and eight studies provided longitudinal data for recurrence-free survival. Forty-three studies were conducted in Asia, 14 studies in America, and 14 studies in Europe.
To investigate the association between HT and sex, 55 studies were analyzed. HT was detected in 802 of 6,489 male (12.4%) and 8,438 of 28,717 female (29.4%) with PTC, and a significant association was identified between female sex and HT (OR, 3.13; 95% CI, 2.67 to 3.68). However, as significant heterogeneity was observed among the studies (I2=65.1%), we conducted analyses of sensitivity and publication bias. The sensitivity analysis identified two outlier studies [1844]. After omitting these studies, the estimated pooled OR was 3.49 (95% CI, 3.21 to 3.79) without significant heterogeneity (I2=49.8%) (Table 2). The funnel plot analysis and the Egger test revealed no significant publication bias (P=0.366).
Thirty-eight studies provided data suitable for a meta-analysis of the association between HT and ETE in patients with PTC. In this population, ETE was reported in 2,856 of 7,311 patients (39.1%) with HT and in 9,796 of 22,527 patients (43.5%) without HT. Accordingly, the coexistence of HT was found to show a negative association with the presence of ETE (OR, 0.75; 95% CI, 0.70 to 0.79) (Table 2). No significant heterogeneity was observed among these studies (I2=30.8%).
For the association between HT and tumor multifocality, 42 studies were included. Among the PTC patients, 2,536 of 8,129 (31.2%) with HT had multifocal disease, as did 6,345 of 22,970 (27.6%) without HT. Coexistent HT and PTC was thus found to be associated with tumor multifocality (OR, 1.17; 95% CI, 1.05 to 1.31) (Table 2). However, as significant heterogeneity was detected among the studies (I2=65%), we conducted analyses of sensitivity and publication bias. After omitting the single outlier study identified in the sensitivity analysis [18], the estimated pooled OR was 1.13 (95% CI, 1.03 to 1.24) without significant heterogeneity (I2=48.6%). The funnel plot analysis and the Egger test revealed no significant publication bias (P=0.741).
To determine the effects on lymph node metastasis, 57 studies were included. Among patients with PTC, 3,829 of 9,767 (39.2%) with HT had lymph node metastasis, as did 12,542 of 29,378 patients (42.7%) without HT. Accordingly, coexistent HT and PTC was found to be negatively associated with lymph node metastasis (OR, 0.82; 95% CI, 0.72 to 0.94) (Table 2). However, as significant heterogeneity was identified among the studies (I2=81%), we analyzed sensitivity and publication bias. In the sensitivity analysis, the pooled ORs ranged from 0.79 to 0.85 after omitting each study individually, and remained statistically significant. The funnel plot analysis and the Egger test revealed no significant publication bias (P=0.647).
Eight studies that included 957 patients with HT and 4,221 patients without HT were analyzed with regard to distant metastasis. Distant metastasis was reported in 25 patients (2.6%) with HT and 185 patients (4.4%) without HT. Accordingly, the coexistence of HT with PTC was found to be negatively correlated with distant metastasis (OR, 0.49; 95% CI, 0.32 to 0.76) (Table 2). No significant heterogeneity was found among these studies (I2=0%).
Nineteen studies, including 11 cross-sectional studies and eight longitudinal studies, provided data suitable for a meta-analysis of the association between HT and PTC recurrence. Here, coexistent HT and PTC were found to be negatively associated with the recurrence of PTC (RR, 0.50; 95% CI, 0.41 to 0.61) (Fig. 2). No significant heterogeneity was found among these studies (I2=0%), and a subgroup analysis by study design yielded similar results (Fig. 2).
We next performed a subgroup analysis according to the mean tumor size (Table 3). Here, the significant negative associations of HT with ETE were independent of the mean tumor size. Among cases with a mean tumor size >1.0 cm, coexistent HT was negatively associated with lymph node and distant metastases and recurrence of PTC.
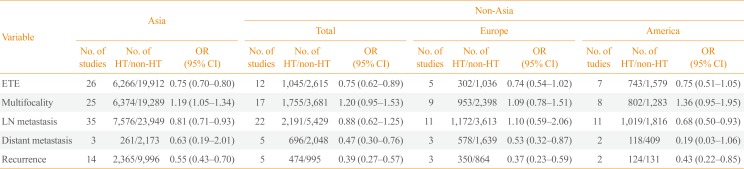
A subgroup analysis based on the study location revealed that the significant associations of HT with absence of ETE and recurrence were location-independent. Among Asian patients, coexistent HT was negatively correlated with lymph node metastasis of PTC, whereas in studies conducted elsewhere, coexistent HT was negatively correlated with distant metastasis (Table 4).
An association between HT and PTC was initially proposed in 1955 by Dailey et al. [79], who linked chronic inflammation to neoplastic changes. Since then, many studies have aimed to evaluate the association between these two prevalent diseases [380]. In this updated meta-analysis of 71 observational studies, we demonstrated that coexistent HT was significantly associated with better clinicopathologic characteristics and an improved prognosis among patients with PTC, as demonstrated by reduced incidence of ETE, lymph node metastasis, and distant metastasis and an increased recurrence-free survival duration relative to those in patients without HT. In contrast to clinicopathologic characteristics, multifocality was positively correlated with HT. Because multifocality has been considered to be a feature associated with the development of PTC, rather than with its aggravation, these findings are consistent with previous studies that reported a positive association of HT with PTC development, along with a protective role of HT against the progression of PTC [356]. Although significant heterogeneity was detected, the results did not change after adjustments via subgroup, sensitivity, and publication bias analyses.
Although the mechanism by which HT affects PTC remains unclear, several hypotheses have been suggested. Among them, inflammation-induced carcinoma has been proposed as one of possible mechanism [81]. The activated inflammatory response in HT, which involves the production of mediators by immune cells in a state of chronic inflammation, might create a favorable setting for malignant transformation in the thyroid gland [381]. However, this hypothesis has difficulties explaining the protective role of HT against the progression of PTC. In this context, tumor defense-induced autoimmunity, such as thyroid-specific cytotoxic T-cells, might play a role [3]. Thyroglobulin (Tg) and thyroid peroxidase (TPO), which are presented on antigen-presenting cells and thyrocytes, are the main target antigens of cellular immune reactions in HT, and these immune reactions may result in target-specific destruction of the thyroid gland [3]. Since TPO and Tg also seem to be specific target antigens in PTC, anti-thyroid antibodies may destroy PTC in the same way as they destroy normal thyroid follicular cells [382]. Several studies have reported increases in antitumor T-cell-mediated immune reactions [798384], while others have suggested the involvement of apoptotic pathway activation [85]. However, whether thyroid-specific cytotoxic T-cells also recognize TPO and Tg in PTC needs investigation.
A genetic predisposition was proposed as another possible mechanism of the protective properties of HT against the progression of PTC, because the BRAF V600E mutation, a marker of more aggressive behavior in PTC, was less frequently detected in patients with coexistent HT than in those without HT [86].
This study had considerable strengths, such as the inclusion of many observational studies with large populations and the performance of predefined subgroup analyses. Our study is therefore the first to demonstrate an association of HT with better outcomes of PTC, regardless of tumor size. Furthermore, we conducted a subgroup analysis according to the region where each study was performed to account for differences in iodine status, and observed that although most studies were performed in Asia and indicated better outcomes of PTC among patients with coexistent HT, studies from non-Asian countries yielded similar results.
Despite the above strengths, however, the present study also had some potential limitations. First, the included studies mainly featured retrospective designs. Further prospective studies are needed to clarify the potential causal relationship between HT and PTC. Second, inter-study differences in age, sex ratio, and iodine status might have led to bias. Third, the included studies predominantly involved Asian populations. Although we performed a subgroup analysis based on study location, further large cohort studies involving multiple races are needed.
In conclusion, this meta-analysis clearly demonstrated that among patients with PTC, coexistent HT is associated with better clinicopathologic features and clinical outcomes. Although the underlying mechanism remains unclear, our findings suggest that this association could be used to predict the prognosis of PTC in clinical settings. Further prospective and large cohort studies are warranted to elucidate the link between HT and PTC.
Notes
AUTHOR CONTRIBUTIONS:
Conception or design: S.M., Y.J.P.
Acquisition, analysis, or interpretation of data: S.M., J.M.Y., H.J.Y., J.H.P., D.S.K., Y.J.P.
Analysis or interpretation of data: H.S.C.
Drafting the work or revising: S.M., Y.J.P.
Final approval of the manuscript: S.M., H.S.C., J.M.Y., H.J.Y., J.H.P., D.S.K., Y.J.P.
Obtained funding, statistical analysis, etc.: S.M.
References
1. Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto thyroiditis: a century later. Adv Anat Pathol. 2012; 19:181–186. PMID: 22498583.
2. Latina A, Gullo D, Trimarchi F, Benvenga S. Hashimoto's thyroiditis: similar and dissimilar characteristics in neighboring areas: possible implications for the epidemiology of thyroid cancer. PLoS One. 2013; 8:e55450. PMID: 23526929.

3. Ehlers M, Schott M. Hashimoto's thyroiditis and papillary thyroid cancer: are they immunologically linked? Trends Endocrinol Metab. 2014; 25:656–664. PMID: 25306886.

4. Choi YM, Kim TY, Jang EK, Kwon H, Jeon MJ, Kim WG, et al. Standardized thyroid cancer mortality in Korea between 1985 and 2010. Endocrinol Metab (Seoul). 2014; 29:530–535. PMID: 25559576.

5. Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto's thyroiditis: a meta-analysis. Eur J Endocrinol. 2013; 168:343–349. PMID: 23211578.

6. Lai X, Xia Y, Zhang B, Li J, Jiang Y. A meta-analysis of Hashimoto's thyroiditis and papillary thyroid carcinoma risk. Oncotarget. 2017; 8:62414–62424. PMID: 28977955.

7. Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD. Well-differentiated thyroid carcinoma with concomitant Hashimoto's thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol. 2011; 22:144–149. PMID: 21647844.

8. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2009; 71:581–586. PMID: 19222495.

9. Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995; 80:3421–3424. PMID: 8530576.

10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010; 25:603–605. PMID: 20652370.

11. Song E, Jeon MJ, Park S, Kim M, Oh HS, Song DE, et al. Influence of coexistent Hashimoto's thyroiditis on the extent of cervical lymph node dissection and prognosis in papillary thyroid carcinoma. Clin Endocrinol (Oxf). 2018; 88:123–128. PMID: 28906015.

12. Cortes MCS, Rosario PW, Mourao GF, Calsolari MR. Influence of chronic lymphocytic thyroiditis on the risk of persistent and recurrent disease in patients with papillary thyroid carcinoma and elevated antithyroglobulin antibodies after initial therapy. Braz J Otorhinolaryngol. 2018; 84:448–452. PMID: 28625809.
13. Zhang Q, Liu BJ, Ren WW, He YP, Li XL, Zhao CK, et al. Association between BRAF V600E mutation and ultrasound features in papillary thyroid carcinoma patients with and without Hashimoto's thyroiditis. Sci Rep. 2017; 7:4899. PMID: 28687736.

14. Sun Y, Liu X, Ouyang W, Feng H, Wu J, Chen P, et al. Lymph node characteristics for predicting locoregional recurrence of papillary thyroid cancer in adolescents and young adults. Oral Oncol. 2017; 66:22–27. PMID: 28249644.

15. Marotta V, Sciammarella C, Chiofalo MG, Gambardella C, Bellevicine C, Grasso M, et al. Hashimoto's thyroiditis predicts outcome in intrathyroidal papillary thyroid cancer. Endocr Relat Cancer. 2017; 24:485–493. PMID: 28696209.

16. Carvalho MS, Rosario PW, Mourao GF, Calsolari MR. Chronic lymphocytic thyroiditis does not influence the risk of recurrence in patients with papillary thyroid carcinoma and excellent response to initial therapy. Endocrine. 2017; 55:954–958. PMID: 27878772.

17. Zhu Y, Zheng K, Zhang H, Chen L, Xue J, Ding M, et al. The clinicopathologic differences of central lymph node metastasis in predicting lateral lymph node metastasis and prognosis in papillary thyroid cancer associated with or without Hashimoto's thyroiditis. Tumour Biol. 2016; 37:8037–8045. PMID: 26711787.

18. Zhu F, Shen YB, Li FQ, Fang Y, Hu L, Wu YJ. The effects of Hashimoto thyroiditis on lymph node metastases in unifocal and multifocal papillary thyroid carcinoma: a retrospective Chinese Cohort Study. Medicine (Baltimore). 2016; 95:e2674. PMID: 26871795.
19. Zeng RC, Jin LP, Chen ED, Dong SY, Cai YF, Huang GL, et al. Potential relationship between Hashimoto's thyroiditis and BRAF(V600E) mutation status in papillary thyroid cancer. Head Neck. 2016; 38(Suppl 1):E1019–E1025. PMID: 26041461.

20. Zeng R, Shou T, Yang KX, Shen T, Zhang JP, Zuo RX, et al. Papillary thyroid carcinoma risk factors in the Yunnan plateau of southwestern China. Ther Clin Risk Manag. 2016; 12:1065–1074. PMID: 27418831.
21. Yu Y, Zhang J, Lu G, Li T, Zhang Y, Yu N, et al. Clinical relationship between IgG4-positive Hashimoto's thyroiditis and papillary thyroid carcinoma. J Clin Endocrinol Metab. 2016; 101:1516–1524. PMID: 26866571.

22. Su X, He C, Ma J, Tang T, Zhang X, Ye Z, et al. RET/PTC rearrangements are associated with elevated postoperative TSH levels and multifocal lesions in papillary thyroid cancer without concomitant thyroid benign disease. PLoS One. 2016; 11:e0165596. PMID: 27802347.

23. Nam HY, Lee HY, Park GC. Impact of co-existent thyroiditis on clinical outcome in papillary thyroid carcinoma with high preoperative serum antithyroglobulin antibody: a retrospective cohort study. Clin Otolaryngol. 2016; 41:358–364. PMID: 26283460.

24. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and pathologic predictors of lymph node metastasis and recurrence in papillary thyroid microcarcinoma. Thyroid. 2016; 26:807–815. PMID: 27117842.

25. Lin X, Chen X, Jiru Y, Du J, Zhao G, Wu Z. Evaluating the influence of prophylactic central neck dissection on TNM staging and the recurrence risk stratification of cN0 differentiated thyroid carcinoma. Bull Cancer. 2016; 103:535–540. PMID: 27236850.

26. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Chronic lymphocytic thyroiditis and BRAF V600E in papillary thyroid carcinoma. Endocr Relat Cancer. 2016; 23:27–34. PMID: 26598713.

27. Kim SJ, Myong JP, Jee HG, Chai YJ, Choi JY, Min HS, et al. Combined effect of Hashimoto's thyroiditis and BRAF (V600E) mutation status on aggressiveness in papillary thyroid cancer. Head Neck. 2016; 38:95–101. PMID: 25213729.
28. Dobrinja C, Makovac P, Pastoricchio M, Cipolat Mis T, Bernardi S, Fabris B, et al. Coexistence of chronic lymphocytic thyroiditis and papillary thyroid carcinoma. Impact on presentation, management, and outcome. Int J Surg. 2016; 28(Suppl 1):S70–S74. PMID: 26708864.

29. Iliadou PK, Effraimidis G, Konstantinos M, Grigorios P, Mitsakis P, Patakiouta F, et al. Chronic lymphocytic thyroiditis is associated with invasive characteristics of differentiated thyroid carcinoma in children and adolescents. Eur J Endocrinol. 2015; 173:827–833. PMID: 26369577.

30. Zhu J, Wang X, Zhang X, Li P, Hou H. Clinicopathological features of recurrent papillary thyroid cancer. Diagn Pathol. 2015; 10:96. PMID: 26168921.

31. Wu Q, Li Y, Wang Y, Hu B. Sonographic features of primary tumor as independent predictive factors for lymph node metastasis in papillary thyroid carcinoma. Clin Transl Oncol. 2015; 17:830–834. PMID: 26041723.

32. Park JY, Kim DW, Park HK, Ha TK, Jung SJ, Kim DH, et al. Comparison of T stage, N stage, multifocality, and bilaterality in papillary thyroid carcinoma patients according to the presence of coexisting lymphocytic thyroiditis. Endocr Res. 2015; 40:151–155. PMID: 25531396.

33. Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, et al. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol. 2015; 20:463–473. PMID: 25312294.

34. Jiwang L, Zhendong L, Shuchun L, Bo H, Yanguo L. Clinicopathologic characteristics of familial versus sporadic papillary thyroid carcinoma. Acta Otorhinolaryngol Ital. 2015; 35:234–242. PMID: 26824209.
35. Girardi FM, Barra MB, Zettler CG. Papillary thyroid carcinoma: does the association with Hashimoto's thyroiditis affect the clinicopathological characteristics of the disease? Braz J Otorhinolaryngol. 2015; 81:283–287. PMID: 25458258.

36. Cai YF, Wang QX, Ni CJ, Guo GL, Li Q, Wang OC, et al. The clinical relevance of Psammoma body and Hashimoto thyroiditis in papillary thyroid carcinoma: a large case-control study. Medicine (Baltimore). 2015; 94:e1881. PMID: 26554782.
37. Zhang Y, Ma XP, Deng FS, Liu ZR, Wei HQ, Wang XH, et al. The effect of chronic lymphocytic thyroiditis on patients with thyroid cancer. World J Surg Oncol. 2014; 12:277. PMID: 25179111.

38. Zhang Y, Dai J, Wu T, Yang N, Yin Z. The study of the coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma. J Cancer Res Clin Oncol. 2014; 140:1021–1026. PMID: 24619663.

39. Yang Y, Chen C, Chen Z, Jiang J, Chen Y, Jin L, et al. Prediction of central compartment lymph node metastasis in papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2014; 81:282–288. PMID: 24483297.

40. Lang BH, Chai YJ, Cowling BJ, Min HS, Lee KE, Youn YK. Is BRAFV600E mutation a marker for central nodal metastasis in small papillary thyroid carcinoma? Endocr Relat Cancer. 2014; 21:285–295. PMID: 24402044.

41. Ryu IS, Song CI, Choi SH, Roh JL, Nam SY, Kim SY. Lymph node ratio of the central compartment is a significant predictor for locoregional recurrence after prophylactic central neck dissection in patients with thyroid papillary carcinoma. Ann Surg Oncol. 2014; 21:277–283. PMID: 24006096.

42. Liu X, Zhu L, Cui D, Wang Z, Chen H, Duan Y, et al. Coexistence of histologically confirmed Hashimoto's thyroiditis with different stages of papillary thyroid carcinoma in a consecutive chinese cohort. Int J Endocrinol. 2014; 2014:769294. PMID: 25505911.

43. Liang K, He L, Dong W, Zhang H. Risk factors of central lymph node metastasis in cN0 papillary thyroid carcinoma: a study of 529 patients. Med Sci Monit. 2014; 20:807–811. PMID: 24831428.

44. Konturek A, Barczynski M, Nowak W, Wierzchowski W. Risk of lymph node metastases in multifocal papillary thyroid cancer associated with Hashimoto's thyroiditis. Langenbecks Arch Surg. 2014; 399:229–236. PMID: 24407910.

45. Kim HG, Kim EK, Han KH, Kim H, Kwak JY. Pathologic spectrum of lymphocytic infiltration and recurrence of papillary thyroid carcinoma. Yonsei Med J. 2014; 55:879–885. PMID: 24954314.

46. Bircan HY, Koc B, Akarsu C, Demiralay E, Demirag A, Adas M, et al. Is Hashimoto's thyroiditis a prognostic factor for thyroid papillary microcarcinoma? Eur Rev Med Pharmacol Sci. 2014; 18:1910–1915. PMID: 25010622.
47. Giagourta I, Evangelopoulou C, Papaioannou G, Kassi G, Zapanti E, Prokopiou M, et al. Autoimmune thyroiditis in benign and malignant thyroid nodules: 16-year results. Head Neck. 2014; 36:531–535. PMID: 23729390.

48. Ye ZQ, Gu DN, Hu HY, Zhou YL, Hu XQ, Zhang XH. Hashimoto's thyroiditis, microcalcification and raised thyrotropin levels within normal range are associated with thyroid cancer. World J Surg Oncol. 2013; 11:56. PMID: 23496874.

49. Shin JH, Ha TK, Park HK, Ahn MS, Kim KH, Bae KB, et al. Implication of minimal extrathyroidal extension as a prognostic factor in papillary thyroid carcinoma. Int J Surg. 2013; 11:944–947. PMID: 23820062.

50. Marotta V, Guerra A, Zatelli MC, Uberti ED, Di Stasi V, Faggiano A, et al. BRAF mutation positive papillary thyroid carcinoma is less advanced when Hashimoto's thyroiditis lymphocytic infiltration is present. Clin Endocrinol (Oxf). 2013; 79:733–738. PMID: 23469895.
51. Kim YS, Choi HJ, Kim ES. Papillary thyroid carcinoma with thyroiditis: lymph node metastasis, complications. J Korean Surg Soc. 2013; 85:20–24. PMID: 23833756.

52. Lim JY, Hong SW, Lee YS, Kim BW, Park CS, Chang HS, et al. Clinicopathologic implications of the BRAF(V600E) mutation in papillary thyroid cancer: a subgroup analysis of 3130 cases in a single center. Thyroid. 2013; 23:1423–1430. PMID: 23496275.

53. Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, et al. Hashimoto's thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg. 2013; 148:396–402. PMID: 23300224.

54. Jara SM, Carson KA, Pai SI, Agrawal N, Richmon JD, Prescott JD, et al. The relationship between chronic lymphocytic thyroiditis and central neck lymph node metastasis in North American patients with papillary thyroid carcinoma. Surgery. 2013; 154:1272–1280. PMID: 24238047.

55. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. 2013; 98:2409–2414. PMID: 23609834.

56. Cordioli MI, Cury AN, Nascimento AO, Oliveira AK, Mello M, Saieg MA. Study of the histological profile of papillary thyroid carcinomas associated with Hashimoto's thyroiditis. Arq Bras Endocrinol Metabol. 2013; 57:445–449. PMID: 24030184.

57. Yoon YH, Kim HJ, Lee JW, Kim JM, Koo BS. The clinicopathologic differences in papillary thyroid carcinoma with or without co-existing chronic lymphocytic thyroiditis. Eur Arch Otorhinolaryngol. 2012; 269:1013–1017. PMID: 21822854.

58. Paulson LM, Shindo ML, Schuff KG. Role of chronic lymphocytic thyroiditis in central node metastasis of papillary thyroid carcinoma. Otolaryngol Head Neck Surg. 2012; 147:444–449. PMID: 22547555.

59. Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, et al. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012; 27:883–889. PMID: 22876054.

60. Campos LA, Picado SM, Guimaraes AV, Ribeiro DA, Dedivitis RA. Thyroid papillary carcinoma associated to Hashimoto's thyroiditis. Braz J Otorhinolaryngol. 2012; 78:77–80. PMID: 23306572.

61. Villagelin DG, Santos RB, Romaldini JH. Is diffuse and peritumoral lymphocyte infiltration in papillary thyroid cancer a marker of good prognosis? J Endocrinol Invest. 2011; 34:e403–e408. PMID: 21765238.
62. Nemetz MA, Thomazelli FC, Granero LC, Nemetz AB, Dos Santos MB. Does chronic lymphocytic thyroiditis influence the staging of differentiated thyroid carcinoma? Braz J Otorhinolaryngol. 2011; 77:77–83. PMID: 21340193.
63. Kim SS, Lee BJ, Lee JC, Kim SJ, Jeon YK, Kim MR, et al. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma: the influence of lymph node metastasis. Head Neck. 2011; 33:1272–1277. PMID: 21837696.

64. Fiore E, Rago T, Latrofa F, Provenzale MA, Piaggi P, Delitala A, et al. Hashimoto's thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer. 2011; 18:429–437. PMID: 21565972.

65. Ahn D, Heo SJ, Park JH, Kim JH, Sohn JH, Park JY, et al. Clinical relationship between Hashimoto's thyroiditis and papillary thyroid cancer. Acta Oncol. 2011; 50:1228–1234. PMID: 21871002.

66. Kim KW, Park YJ, Kim EH, Park SY, Park DJ, Ahn SH, et al. Elevated risk of papillary thyroid cancer in Korean patients with Hashimoto's thyroiditis. Head Neck. 2011; 33:691–695. PMID: 21484918.

67. Mazokopakis EE, Tzortzinis AA, Dalieraki-Ott EI, Tsartsalis AN, Syros PK, Karefilakis CM, et al. Coexistence of Hashimoto's thyroiditis with papillary thyroid carcinoma: a retrospective study. Hormones (Athens). 2010; 9:312–317. PMID: 21112862.

68. Consorti F, Loponte M, Milazzo F, Potasso L, Antonaci A. Risk of malignancy from thyroid nodular disease as an element of clinical management of patients with Hashimoto's thyroiditis. Eur Surg Res. 2010; 45:333–337. PMID: 21051899.

69. Muzza M, Degl'Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf). 2010; 72:702–708. PMID: 20447069.

70. Kim HS, Choi YJ, Yun JS. Features of papillary thyroid microcarcinoma in the presence and absence of lymphocytic thyroiditis. Endocr Pathol. 2010; 21:149–153. PMID: 20506003.

71. French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. 2010; 95:2325–2333. PMID: 20207826.

72. Gul K, Dirikoc A, Kiyak G, Ersoy PE, Ugras NS, Ersoy R, et al. The association between thyroid carcinoma and Hashimoto's thyroiditis: the ultrasonographic and histopathologic characteristics of malignant nodules. Thyroid. 2010; 20:873–878. PMID: 20677997.

73. Kim SK, Song KH, Lim SD, Lim YC, Yoo YB, Kim JS, et al. Clinical and pathological features and the BRAF(V600E) mutation in patients with papillary thyroid carcinoma with and without concurrent Hashimoto thyroiditis. Thyroid. 2009; 19:137–141. PMID: 19014278.

74. Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, et al. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007; 204:764–773. PMID: 17481480.

75. Kurukahvecioglu O, Taneri F, Yuksel O, Aydin A, Tezel E, Onuk E. Total thyroidectomy for the treatment of Hashimoto's thyroiditis coexisting with papillary thyroid carcinoma. Adv Ther. 2007; 24:510–516. PMID: 17660159.

76. Kebebew E, Treseler PA, Ituarte PH, Clark OH. Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg. 2001; 25:632–637. PMID: 11369991.

77. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999; 126:1070–1076. PMID: 10598190.

78. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, et al. Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid. 1998; 8:197–202. PMID: 9545105.

79. Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955; 70:291–297. PMID: 13227748.

80. Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto's thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013; 98:474–482. PMID: 23293329.
81. Liotti F, Visciano C, Melillo RM. Inflammation in thyroid oncogenesis. Am J Cancer Res. 2012; 2:286–297. PMID: 22679559.
82. Latrofa F, Ricci D, Grasso L, Vitti P, Masserini L, Basolo F, et al. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. J Clin Endocrinol Metab. 2008; 93:591–596. PMID: 18029466.

83. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999; 84:458–463. PMID: 10022401.

84. Lucas SD, Karlsson-Parra A, Nilsson B, Grimelius L, Akerstrom G, Rastad J, et al. Tumor-specific deposition of immunoglobulin G and complement in papillary thyroid carcinoma. Hum Pathol. 1996; 27:1329–1335. PMID: 8958307.

85. Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, et al. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science. 1997; 275:960–963. PMID: 9020075.

86. Zhang Q, Liu SZ, Zhang Q, Guan YX, Chen QJ, Zhu QY. Meta-analyses of association between BRAF(V600E) mutation and clinicopathological features of papillary thyroid carcinoma. Cell Physiol Biochem. 2016; 38:763–776. PMID: 26871894.

Fig. 2
Forest plots summarizing the risk ratio of the association between Hashimoto thyroiditis and recurrence of papillary thyroid cancer. RR, relative risk; CI, confidence interval.
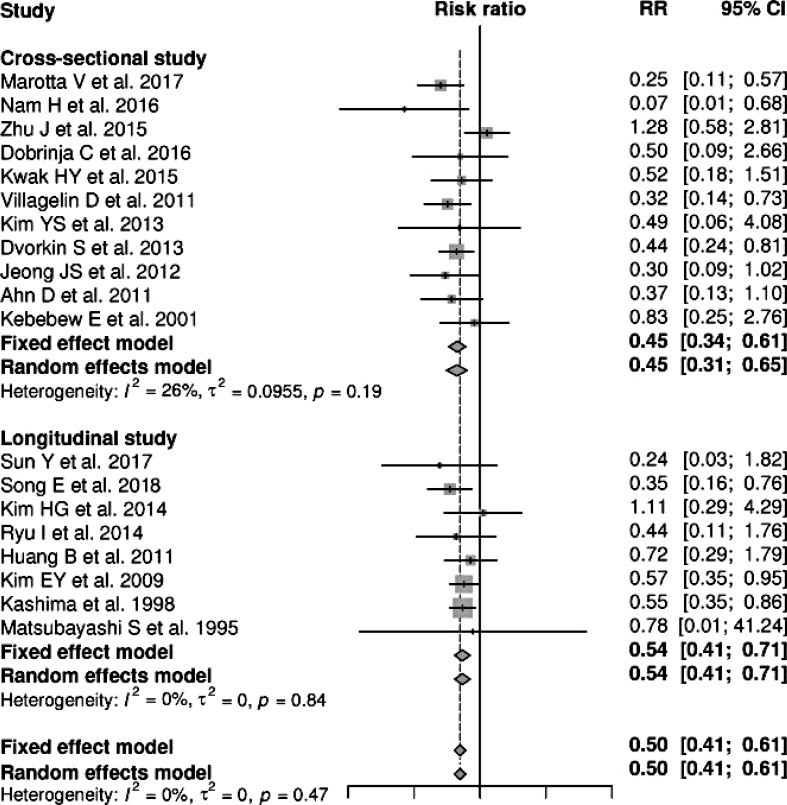
Table 1
Summary of the 71 Studies Included in the Present Meta-Analysis
| Study | Country | No. of HT/ PTC (%) | Sex ratio (male), % | Sex | Multifocality | ETE | Lymph node metastasis | Distant metastasis | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| Song et al. (2018) [11] | Korea | 305/1,369 (22.3) | 16.9 | Y | Y | Y | Y | Ya | |
| Cortes et al. (2018) [12] | Brazil | 45/113 (39.8) | 8.8 | Y | Y | ||||
| Zhang et al. (2017) [13] | China | 163/438 (37.2) | 22.8 | Y | |||||
| Sun et al. (2017) [14] | China | 60/329 (18.2) | Ya | ||||||
| Marotta et al. (2017) [15] | Italy | 173/301 (57.5) | 15.9 | Y | Y | Y | |||
| Carvalho et al. (2017) [16] | Brazil | 191/633 (30.2) | 19.9 | Y | Y | Y | Y | ||
| Zhu et al. (2016) [17] | China | 313/1,276 (24.5) | 20.3 | Y | Y | Y | Y | ||
| Zhu et al. (2016) [18] | China | 277/763 (36.3) | 24.2 | Y | Y | Y | |||
| Zeng et al. (2016) [19] | China | 222/619 (35.9) | 21.8 | Y | Y | Y | Y | ||
| Zeng et al. (2016) [20] | China | 70/578 (12.1) | 15.9 | Y | Y | Y | |||
| Yu et al. (2016) [21] | China | 48/66 (72.7) | 12.1 | Y | |||||
| Su et al. (2016) [22] | China | 56/114 (49.1) | Y | Y | Y | ||||
| Nam et al. (2016) [23] | Korea | 22/37 (59.5) | 16.2 | Y | Y | Y | Y | Y | Y |
| Siddiqui et al. (2016) [24] | USA | 93/163 (57.1) | Y | ||||||
| Lin et al. (2016) [25] | China | 26/153 (17) | Y | ||||||
| Kim et al. (2016) [26] | Korea | 1,006/3,332 (30.2) | 22 | Y | Y | Y | Y | ||
| Kim et al. (2016) [27] | Korea | 204/1,780 (11.5) | 16.5 | Y | Y | Y | Y | ||
| Dobrinja et al. (2016) [28] | Italy | 70/160 (43.8) | 32.5 | Y | Y | Y | |||
| Illiadou et al. (2015) [29] | Greece | 31/108 (28.7) | 24.1 | Y | Y | Y | Y | Y | |
| Zhu et al. (2015) [30] | China | 79/315 (25.1) | Y | ||||||
| Wu et al. (2015) [31] | China | 64/284 (22.5) | Y | ||||||
| Park et al (2015) [32] | Korea | 169/653 (25.9) | 13.9 | Y | Y | Y | |||
| Kwak et al. (2015) [33] | Korea | 452/1,945 (23.2) | 17.8 | Y | Y | Y | Y | ||
| Jiwang et al. (2015) [34] | China | 5/24 (20.8) | 37.5 | Y | Y | Y | Y | ||
| Girardi et al. (2015) [35] | Brazil | 148/417 (35.5) | 18.7 | Y | Y | Y | Y | ||
| Cai et al. (2015) [36] | China | 229/1,052 (21.8) | Y | Y | Y | ||||
| Zhang et al. (2014) [37] | China | 44/144 (30.6) | 26.4 | Y | Y | Y | |||
| Zhang et al. (2014) [38] | China | 247/1,735 (14.2) | Y | Y | Y | ||||
| Yang et al. (2014) [39] | China | 92/291 (31.6) | Y | ||||||
| Lang et al. (2014) [40] | Korea | 331/745 (44.4) | Y | ||||||
| Ryu et al. (2014) [41] | Korea | 49/283 (17.3) | Ya | ||||||
| Liu et al. (2014) [42] | China | 581/1,722 (33.7) | 20.2 | Y | Y | ||||
| Liang et al. (2014) [43] | China | 116/529 (21.9) | Y | ||||||
| Konturek et al. (2014) [44] | Poland | 130/773 (16.8) | 11.5 | Y | Y | Y | |||
| Kim et al. (2014) [45] | Korea | 41/144 (28.5) | 7.6 | Y | Y | Y | Y | Ya | |
| Bircan et al. (2014) [46] | Turkey | 67/172 (39) | 16.9 | Y | Y | Y | |||
| Giagourta et al. (2014) [47] | Greece | 441/1,380 (32) | Y | Y | Y | ||||
| Ye et al. (2013) [48] | China | 187/1,004 (18.6) | 17.5 | Y | Y | Y | Y | ||
| Shin et al. (2013) [49] | Korea | 64/332 (19.3) | Y | ||||||
| Marotta et al. (2013) [50] | Italy | 54/146 (37) | 20.5 | Y | Y | Y | Y | ||
| Kim et al. (2013) [51] | Korea | 316/1,247 (25.3) | 13.2 | Y | Y | Y | Y | Y | |
| Lim et al. (2013) [52] | Korea | 964/2,947 (32.7) | 20.3 | Y | Y | Y | Y | ||
| Lun et al. (2013) [53] | China | 127/676 (18.8) | 20.6 | Y | Y | ||||
| Jara et al. (2013) [54] | USA | 226/495 (45.7) | 21 | Y | Y | Y | Y | ||
| Dvorkin et al. (2013) [55] | Israel | 107/753 (14.2) | Y | Y | Y | Y | |||
| Cordioli et al. (2013) [56] | Brazil | 35/94 (37.2) | 14.9 | Y | Y | Y | |||
| Yoon et al. (2012) [57] | Korea | 56/195 (28.7) | 14.9 | Y | Y | Y | Y | ||
| Paulson et al. (2012) [58] | USA | 61/139 (43.9) | 20.1 | Y | Y | Y | Y | Y | |
| Jeong et al. (2012) [59] | Korea | 359/1,357 (26.5) | 13.3 | Y | Y | Y | Y | Y | |
| Campos et al. (2012) [60] | Brazil | 11/41 (26.8) | 17.1 | Y | |||||
| Villagelin et al. (2011) [61] | Brazil | 83/119 (69.7) | 13.4 | Y | Y | Y | Y | ||
| Nemetz et al. (2011) [62] | Brazil | 17/52 (32.7) | 7.7 | Y | Y | Y | |||
| Kim et al. (2011) [63] | Korea | 146/400 (36.5) | 10.8 | Y | Y | Y | Y | Y | |
| Huang et al. (2011) [7] | China | 93/1,997 (4.7) | 17 | Y | Y | Y | Y | ||
| Fiore et al. (2011) [64] | Italy | 112/666 (16.8) | 26.7 | Y | Y | ||||
| Ahn et al. (2011) [65] | Korea | 58/269 (21.6) | 16.4 | Y | Y | Y | Y | Y | |
| Kim et al. (2011) [66] | Korea | 307/1,028 (29.9) | 20.1 | Y | Y | Y | Y | ||
| Mazokopakis et al. (2010) [67] | Greece | 12/32 (37.5) | 21.9 | Y | |||||
| Consorti et al. (2010) [68] | Italy | 25/101 (24.8) | 23.8 | Y | Y | Y | |||
| Muzza et al. (2010) [69] | Italy | 128/343 (37.3) | 21.9 | Y | Y | ||||
| Kim et al. (2010) [70] | Korea | 105/323 (32.5) | 15.2 | Y | Y | Y | |||
| French et al. (2010) [71] | USA | 37/76 (48.7) | 18.4 | Y | Y | ||||
| Gul et al. (2010) [72] | Turkey | 40/171 (23.4) | Y | Y | Y | ||||
| Kim et al. (2009) [73] | Korea | 37/101 (36.6) | 10.9 | Y | Y | Y | |||
| Kim et al. (2009) [8] | Korea | 214/1,441 (14.9) | 13 | Y | Y | Y | Y | Ya | |
| Larson et al. (2007) [74] | USA | 43/198 (21.7) | 21.2 | Y | Y | Y | |||
| Kurukahvecioglu et al. (2007) [75] | Turkey | 37/199 (18.6) | 20.1 | Y | Y | Y | |||
| Kebebew et al. (2001) [76] | USA | 41/136 (30.1) | 30.1 | Y | Y | Y | |||
| Singh et al. (1999) [77] | USA | 57/388 (14.7) | Y | Y | Y | ||||
| Kashima et al. (1998) [78] | Japan | 281/1,533 (18.3) | Y | Y | Y | Ya | |||
| Matsubayashi et al. (1995) [9] | Japan | 36/95 (37.9) | 7.4 | Y | Y | Ya |
Table 2
Meta-Analyses of the Associations between HT and Clinicopathological Features of Papillary Thyroid Cancer
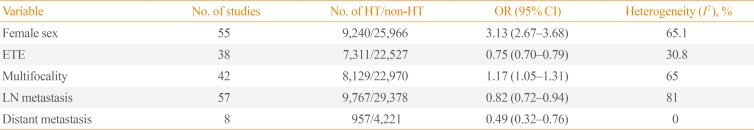
Table 3
Subgroup Analysis of the Associations between HT and Clinicopathological Features of Papillary Thyroid Cancer by Mean Tumor Size
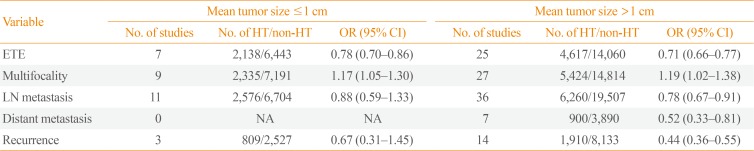
Table 4
Subgroup Analysis of the Associations between HT and Clinicopathological Features of Papillary Thyroid Cancer by Study Location




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download