Abstract
Background
Thyroid dysfunction is associated with negative neonatal and obstetric outcomes. Large differences in thyroid function reference intervals exist across different populations. These differences can be explained by population-specific factors, such as iodine status. Many countries in Latin America report iodine sufficiency, but relatively few countries have published up-to-date data on iodine levels and thyroid function in the overall population, and especially in pregnant women. We evaluated the iodine status of pregnant women in Chile and determined thyroid hormone reference ranges in this population.
Methods
This was a prospective observational study of healthy Chilean women at their first prenatal visit before week 14. Thyroid-stimulating hormone (TSH), total thyroxine (T4), free T4, antithyroid peroxidase antibody (TPOAb), and iodine levels from spot urine samples were measured. Iodine status and the reference ranges for TSH were calculated.
Results
A total of 1,022 pregnant women in the first trimester were selected. Urinary iodine levels were measured in 302 randomly-selected women. The median urinary iodine concentration was 173.45 µg/L (interquartile range, 108.11 to 249.35).The reference ranges of TSH were calculated in 670 patients selected according to the National Academy of Clinical Biochemistry guidelines. The median TSH level was 1.88 µIU/mL (2.5th percentile: 0.13 to 97.5th percentile: 5.37). Using the reference range in the 1,022 women, the prevalence of clinical hypothyroidism was 1.76%, and that of subclinical hypothyroidism was 3.92%. TPOAb positivity was more common in women with TSH levels above 3.5 µIU/mL.
Currently, universal agreement exists about the relevance of maternal thyroid status; however, difficulties remain in establishing reference ranges for maternal thyroid parameters [123].
The currently available thyroid-stimulating hormone (TSH) reference intervals for pregnant women show substantial differences among countries [45678]. According to the National Academy of Clinical Biochemistry (NACB) guidelines, the definition of the reference population includes subjects with no prior history of thyroid disease, no history of taking medication that could influence thyroid function, no family history of thyroid disease, negative antithyroid peroxidase antibody (TPOAb) results, and serum free thyroxine (fT4) levels in the reference range [9]. Current American Thyroid Association (ATA) guidelines also suggest that intervals maybe calculated locally, and that normal ranges might be obtained in a population with normal iodine levels [101112]. However, iodine status is not considered when defining the reference population. The use of iodized salt is not widespread, and it has been established that TSH levels are directly related to the degree of iodine nutrition of the population [13]; therefore, when establishing TSH reference levels, it is necessary to know the iodine status of the population.
The World Health Organization (WHO) has requested that countries perform iodine monitoring, but few studies have been conducted among pregnant women [101112]. A Chinese study recently reported that women with excessive iodine intake had higher mean TSH and lower fT4 concentrations [1415]. In Korea, a recent nationwide survey demonstrated more than adequate iodine intake and a right-shifted distribution of serum TSH levels, also supporting the argument that nutritional iodine status is important for determining serum TSH levels and is associated with a high prevalence of hypothyroidism [16].
Iodination in Chile started in 1979 with a policy of iodination of culinary salt to control iodine deficiency in the population. The Chilean Universal Salt Iodination Program required each kilogram of salt to be fortified with 100 µg of iodine (100 ppm). In the year 2000, measurements showed a very high level of iodine in school-aged children, which motivated a reduction of iodination to 40 ppm [1517], so it is possible that Chileans were exposed to a period of excessive iodine intake. Iodine nutrition status has not subsequently been assessed in Chile.
We conducted the present study to assess iodine status in pregnant Chilean woman and to estimate the local thyroid hormone reference ranges.
A total of 1,038 pregnant women with singleton pregnancies attending their first prenatal care visit at three primary health care centers in central Chile were invited to participate in this study between March 2014 and December 2016. The patients were recruited at a primary care clinic in Curicó (including rural and urban low-socioeconomic-status populations), Red de Salud UC-Christus in Santiago (urban middle-socioeconomic-status women), and a primary care clinic in the southeast sector of Santiago (urban low-socioeconomic-status women). We excluded women with multiple gestations; women who presented to their first prenatal visit after week 14; women with a prior history of thyroid dysfunction, diabetes, or the use of medications that can affect thyroid function, such as antidepressants, anticonvulsants, and antipsychotic drugs; and women with a history of thyroid surgery or radioactive iodine treatment.
This study was approved (01-179) by the Research Ethics Committee of the School of Medicine of Pontificia Universidad Católica de Chile and by the South Metropolitan Health Services Ethics Committee. All participants provided written informed consent.
Gestational age was determined by the last menstrual period and confirmed by ultrasound examination. When a major discrepancy between these 2 dates was found, the date of gestation was ultimately defined by the ultrasound examination. Each participant completed a standardized clinical questionnaire on demographic and obstetric characteristics, with a special emphasis on the thyroid disease risk factors described by the Endocrine Society and the ATA guidelines [34].
In all included patients, a morning fasting venous blood sample of 20 mL and a 50-mL isolated spot urine sample to assess urinary iodine (UI) levels were obtained and kept at 4℃. In all included patients, serum concentrations of TSH, total T4, fT4, and TPOAb were centrally analyzed at the Clinical Laboratory Services of Red de Salud UC in Santiago, which has international accreditation (ISO 15189). UI was determined in a subgroup of 302 patients who were representative of the broader sample.
Ultrasensitive TSH, total T4, and fT4 measurements were made by competitive electrochemiluminescent immunoassay (Modular Analytics E 170, Roche, Indianapolis, IN, USA). For TSH, the threshold of detection was 0.005 µIU/mL, and the intra- and inter-assay coefficients of variation were <3.3% in the range of 0.035 to 3.66 mIU/L. For total T4, the analytical sensitivity was 5.4 nmol/L with intra and inter-assay coefficients of variation <4.2% in the range of 65.64 to 231.66 nmol/L. For fT4, the analytical sensitivity was 0.296 pmol/L with intra- and inter-assay coefficients of variation <3.6% in the range of 14.93 to 25.74 pmol/L. The quantitative determination of TPOAb was performed by a microparticle enzyme immunoassay (AXSYM-ABBOT, Abbott Laboratories, Lake Bluff, IL, USA) with an analytical sensitivity of 1 kIU/L. During the study period, the manufacturer's reference values for non-pregnant women were as follows: TSH 0.3 to 4.2 mIU/L; T4 59.2 to 154.44 nmol/L; fT4 0.8 to 2.0 ng/dL in those <20 years old and 0.93 to 1.7 ng/dL in those >20 years old; and TPOAb <12 kIU/L.
UI was determined by the method of oxide reduction with ammonium persulfate and colorimetric determination at 420 nm at the Clinical Laboratory Services of Red de Salud UC in Santiago, which maintains international cross-lab analyses and transformation factors with central labs in Helsinki [18].
In pregnant women, we considered iodine intake to be insufficient if UI was <150 µg/L, adequate if UI was between 150 and 249 µg/L, more than adequate if UI was between 250 and 499 µg/L and excess if UI was >500 µg/L [19].
The reference population was defined according the NACB guidelines as subjects with no prior history of thyroid disease, no history of taking medication that could influence thyroid function, no family history of thyroid disease, negative TPOAb results, and serum fT4 levels in the reference range. The TSH reference range was estimated from the reference population [9].
Normality tests were applied (Kolmogorov-Smirnov test) to quantitative data. Reference ranges for TSH were estimated excluding women that did not meet NACB criteria. We also exclude atypical values of TSH (i.e., observations that fell outside of the quartile 1 to 3 interquartile range). For a better understanding of the results, tables and graphs are presented. We calculated the median TSH level and the 2.5th and 97.5th percentiles. Logistic regression was performed to identify associations between TPOAb positivity and TSH levels.
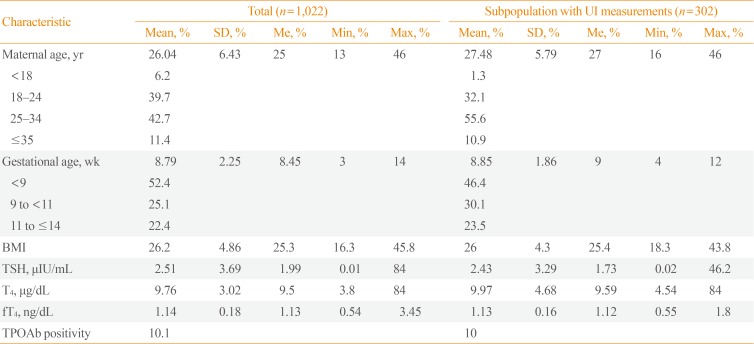
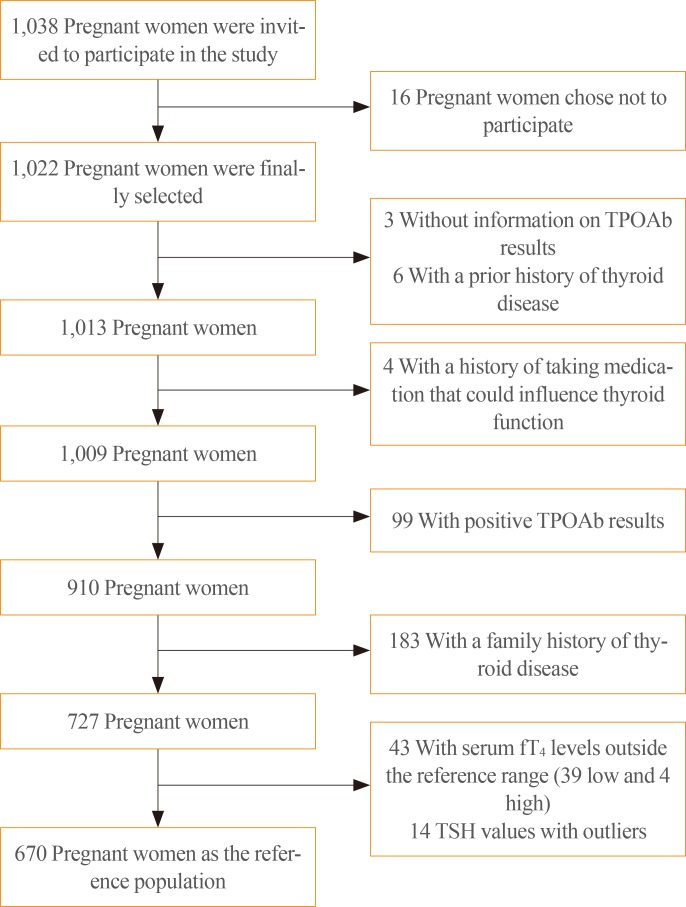
A total of 1,022 first-trimester pregnant women were ultimately selected to participate in the study (Table 1). Sixteen women opted not to participate, and after the selection process, 670 patients formed the reference population (Fig. 1). It was not possible to analyze UI levels in all patients due to financial constraints, so UI levels were measured in a random sample of 302 pregnant women in the first trimester of pregnancy.
The participants in whom UI levels were and were not measured were compared by age, body mass index, TSH, and gestational age, and no statistically significant differences were found, except in age, for which median values of 25 years and 27 years were found in the respective populations.
In the patients selected for UI measurements, the median UI concentration was 173.45 µg/L (interquartile range, 108.11 to 249.35). The distribution of UI levels is shown in Fig. 2.
In the reference population, the mean TSH level was 1.88 µIU/mL, and the 2.5th and 97.5th percentiles were 0.13 and 5.37 µIU/mL, respectively.
Using our reference ranges, we calculated the prevalence of subclinical and clinical hypothyroidism in the total group of 1,022 pregnant women. The prevalence of subclinical hypothyroidism was 3.92%, and that of clinical hypothyroidism was 1.76%. Fig. 3 presents the prevalence of clinical and subclinical hypothyroidism using other TSH cut-off points.
TPOAb was present in 10% of our pregnant population, similar to the findings that have been previously reported in the literature. We evaluated the prevalence of positive TPOAb according to TSH levels in all pregnant women. TPOAb positivity was significantly more common in women with TSH levels higher than 3.5 mIU/L, especially in those with TSH levels above 5.0 mIU/L (Fig. 4).
Hypothyroidism was found to be common in first-trimester pregnant women living in Chile, affecting 5.68% of the pregnant women in our sample. The Chilean iodine fortification program has achieved the adequate recommended iodine intake for pregnant women [1112].
Most Chileans consume sufficient iodine, but iodine sufficiency and excess iodine intake may coexist in our population [20], similar to other countries in Latin America, as described by Mioto et al. [21] in Brazil. In any case, since the population of Chile is classified as iodine-sufficient, spot urine samples should not be used for the purposes of individual diagnosis and treatment.
One of the strengths of this study is the determination of reference thyroid parameters, which have not been previously described for this population in our country.
We applied the reference range calculated for TSH in our population and compared it with other cut-off points described in the literature. Our upper cut-off TSH level (97.5th percentile) was 5.37 µIU/mL, which is higher than the normal ranges reported in the literature for other populations [6222324]. With this level, the calculated prevalence of hypothyroidism in pregnancy was 5.68%, which is higher than has been reported in the literature [25], but significantly lower than the prevalence that would be obtained if we applied the internationally proposed parameters (Fig. 3). In fact, we established a pregnancy-specific reference range for our population in this study with the specific goal of avoiding applying the international reference ranges of TSH, as the ATA and other experts have recommended [1026]. It has been established that using the reference ranges suggested in the 2011 ATA guidelines leads to an elevated prevalence of high TSH (8% to 28%), because in most studies the upper TSH reference range used was higher than 2.5 or 3.0 mIU/L, whereas studies conducted to establish local reference ranges in iodine-replete areas found upper TSH reference values for first- and second-trimester women ranging from 4.04 to 4.34 mIU/L [26]. Those reference ranges are much closer to the values calculated in our population.
It is possible that some determinants such as ethnic factors, environmental factors, or obesity as a comorbidity in our patients could have influenced the upper TSH reference value that we obtained [827].
The possibility of thyroid autoimmune phenomena would not account for the findings in our population, since we did not find a higher prevalence of TPOAb than has been described globally [1112].
It has been proposed that the most appropriate “normal” TSH level might be found in the population with the lowest prevalence of TPOAb [28]. In our analysis of TPOAb-positive patients, we found that TPOAb positivity was significantly more common in patients with TSH levels above 3.5 µIU/mL and further increased with even higher TSH levels; this fact supports the use of a cut-off level over 3.5 µIU/mL as a reference.
A limitation of our study is that the participants in whom UI was measured showed a statistically significant difference in age from those in whom UI was not measured, despite the fact that they were randomly selected; however, since the difference was very small, we do not believe that this age difference was relevant to our findings for the other variables or that it interfered with our global analysis. Furthermore, we did not measure 24-hour UI levels, but the spot urine technique has been supported by the WHO [19]. As a third limitation, it should be noted that measuring thyroid hormones only in the first trimester does not allow us to infer that our calculated ranges are applicable throughout pregnancy.
Population-wide mandatory iodination must be supported. Precautions must be taken to include the lowest amount of iodine necessary to minimize iodine-deficiency disorders, while simultaneously preventing overexposure. Iodine deficiency and overexposure will always coexist; Gauss curves assure this. To avoid overexposure, the fortification of salt should be decreased, but at the same time, strategies targeting subgroups at the highest risk of deficiency must be introduced.
Nutritional iodine status should considered when establishing TSH reference intervals of populations in iodine-replete areas, in which insufficiency and excessive iodine intake coexist in pregnant women.
References
1. Abalovich M, Amino N, Barbour LA, Cobin RH, De Groot LJ, Glinoer D, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2007; 92(8 Suppl):S1–S47. PMID: 17948378.

2. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011; 21:1081–1125. PMID: 21787128.

3. Qaseem A, Snow V, Owens DK, Shekelle P. The development of clinical practice guidelines and guidance statements of the American College of Physicians: summary of methods. Ann Intern Med. 2010; 153:194–199. PMID: 20679562.

4. Orito Y, Oku H, Kubota S, Amino N, Shimogaki K, Hata M, et al. Thyroid function in early pregnancy in Japanese healthy women: relation to urinary iodine excretion, emesis, and fetal and child development. J Clin Endocrinol Metab. 2009; 94:1683–1688. PMID: 19258403.

5. Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester- and method-specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf). 2011; 74:262–269. PMID: 21044115.

6. Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008; 115:602–606. PMID: 18333941.

7. Moon HW, Chung HJ, Park CM, Hur M, Yun YM. Establishment of trimester-specific reference intervals for thyroid hormones in Korean pregnant women. Ann Lab Med. 2015; 35:198–204. PMID: 25729721.

8. Mosso L, Martinez A, Rojas MP, Margozzini P, Solari S, Lyng T, et al. Frequency of subclinical thyroid problems among women during the first trimester of pregnancy. Rev Med Chil. 2012; 140:1401–1408. PMID: 23677185.
9. Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003; 13:3–126. PMID: 12625976.
10. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97:2543–2565. PMID: 22869843.

11. Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European Thyroid Association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014; 3:76–94. PMID: 25114871.

12. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017; 27:315–389. PMID: 28056690.

13. Li C, Shan Z, Mao J, Wang W, Xie X, Zhou W, et al. Assessment of thyroid function during first-trimester pregnancy: what is the rational upper limit of serum TSH during the first trimester in Chinese pregnant women? J Clin Endocrinol Metab. 2014; 99:73–79. PMID: 24276458.

14. Shi X, Han C, Li C, Mao J, Wang W, Xie X, et al. Optimal and safe upper limits of iodine intake for early pregnancy in iodine-sufficient regions: a cross-sectional study of 7190 pregnant women in China. J Clin Endocrinol Metab. 2015; 100:1630–1638. PMID: 25629356.

15. Council on Environmental Health. Rogan WJ, Paulson JA, Baum C, Brock-Utne AC, Brumberg HL, et al. Iodine deficiency, pollutant chemicals, and the thyroid: new information on an old problem. Pediatrics. 2014; 133:1163–1166. PMID: 24864180.
16. Kim WG, Kim WB, Woo G, Kim H, Cho Y, Kim TY, et al. Thyroid stimulating hormone reference range and prevalence of thyroid dysfunction in the Korean population: Korea National Health and Nutrition Examination Survey 2013 to 2015. Endocrinol Metab (Seoul). 2017; 32:106–114. PMID: 28116874.

17. Muzzo S, Burgueno M, Carvajal F, Biolley E, Avendano M, Vargas S, et al. Iodine nutrition in school children of four census areas of Chile. Rev Med Chil. 1997; 125:1299–1304. PMID: 9609050.
18. EUthyroid. Laboratory method and statistical analysis for cross-lab comparison [Internet]. Vienna: Biolution GmbH;c2015. cited 2018 Nov 5. Available from: http://euthyroid.eu/download/EUthyroid_iodinemap_textEN.pdf.
19. World Health Organization. Assessment of iodine deficiency disorders and monitoring their elimination. 3rd ed. Geneva: World Health Organization;2007.
20. Muzzo S, Leiva L, Ramirez I, Carvajal F, Biolley E. Iodine nutrition status in school age children of an area of high iodine intake (Calama) compared with an area of normal intake (Punta Arenas). Rev Chil Nutr. 2005; 32:28–35.
21. Mioto VCB, Monteiro ACCNG, de Camargo RYA, Borel AR, Catarino RM, Kobayashi S, et al. High prevalence of iodine deficiency in pregnant women living in adequate iodine area. Endocr Connect. 2018; 7:762–767. PMID: 29700098.

22. Medici M, Korevaar TI, Visser WE, Visser TJ, Peeters RP. Thyroid function in pregnancy: what is normal? Clin Chem. 2015; 61:704–713. PMID: 25829408.

23. Soldin OP, Tractenberg RE, Hollowell JG, Jonklaas J, Janicic N, Soldin SJ. Trimester-specific changes in maternal thyroid hormone, thyrotropin, and thyroglobulin concentrations during gestation: trends and associations across trimesters in iodine sufficiency. Thyroid. 2004; 14:1084–1090. PMID: 15650363.

24. Quinn FA, Gridasov GN, Vdovenko SA, Krasnova NA, Vodopianova NV, Epiphanova MA, et al. Prevalence of abnormal thyroid stimulating hormone and thyroid peroxidase antibody-positive results in a population of pregnant women in the Samara region of the Russian Federation. Clin Chem Lab Med. 2005; 43:1223–1226. PMID: 16232090.

25. Stricker R, Echenard M, Eberhart R, Chevailler MC, Perez V, Quinn FA, et al. Evaluation of maternal thyroid function during pregnancy: the importance of using gestational age-specific reference intervals. Eur J Endocrinol. 2007; 157:509–514. PMID: 17893266.

26. Korevaar TIM. The upper limit for TSH during pregnancy: why we should stop using fixed limits of 2.5 or 3.0 mU/l. Thyroid Res. 2018; 11:5. PMID: 29942352.

27. Mosso L, Martinez A, Rojas MP, Latorre G, Margozzini P, Lyng T, et al. Early pregnancy thyroid hormone reference ranges in Chilean women: the influence of body mass index. Clin Endocrinol (Oxf). 2016; 85:942–948. PMID: 27260560.

28. Spencer CA, Hollowell JG, Kazarosyan M, Braverman LE. National Health and Nutrition Examination Survey III thyroid-stimulating hormone (TSH)-thyroperoxidase antibody relationships demonstrate that TSH upper reference limits may be skewed by occult thyroid dysfunction. J Clin Endocrinol Metab. 2007; 92:4236–4240. PMID: 17684054.

Fig. 2
Urinary iodine levels during the first trimester of pregnancy. Median urinary iodine level: 173.45 µg/L.
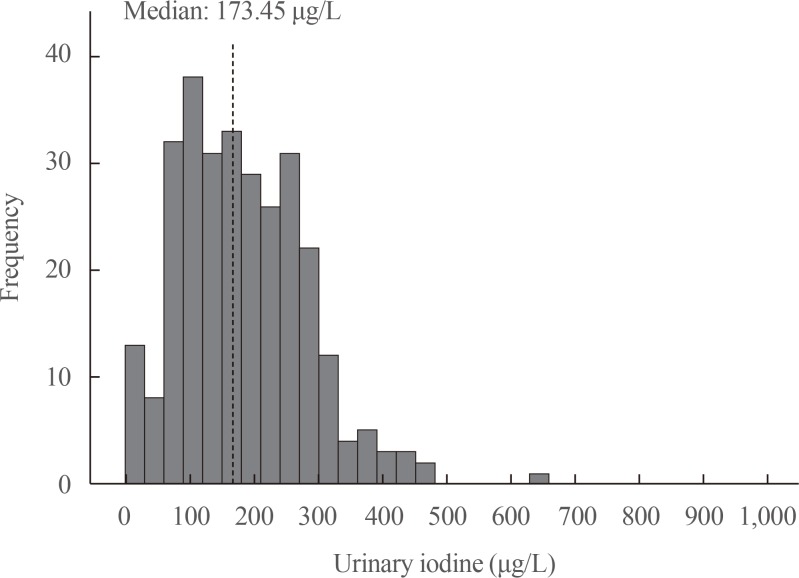
Fig. 3
Distribution of thyroid-stimulating hormone (TSH) levels (µIU/mL) in the first trimester of pregnancy in 1,022 women and differences in the prevalence of hypothyroidism using different cut-off points (2.5, 4.2, and 5.36 µIU/mL).
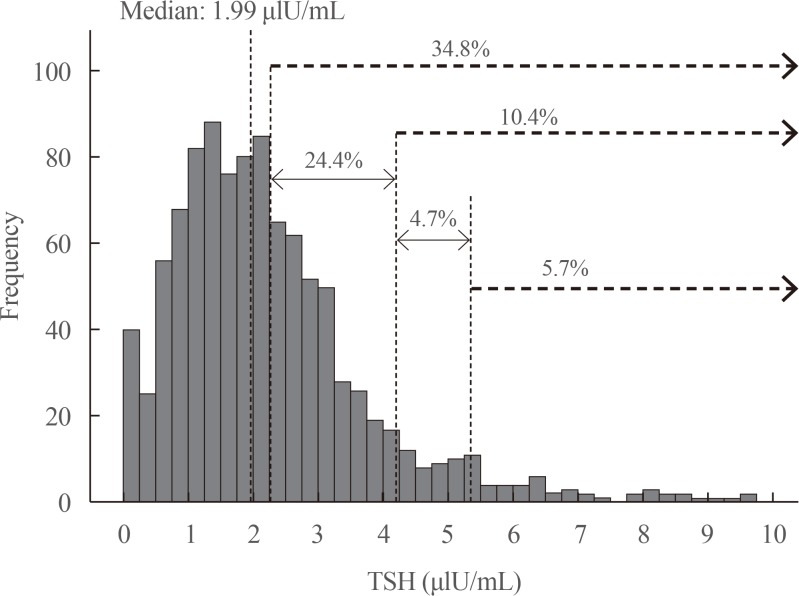
Fig. 4
Prevalence of positive antithyroid peroxidase antibody (TPOAb) according to thyroid-stimulating hormone (TSH) levels in pregnant women during the first trimester. aSignificant differences of anti-TPOAb prevalence with respect to the lowest prevalence within the normal range (5.66%; 95% confidence interval [CI], 2.03 to 9.29) in 1.51 to 2.0 TSH category. Prevalence in 3.01 to 3.50, 3.51 to 4.0, 4.01 to 4.50, 5.01 to 7.50, 7.51 to 10.00, and 10.01+; TSH groups are 13.16% (95% CI, 5.38 to 20.93), 17.02% (95% CI, 5.87 to 28.18), 22.22% (95% CI, 5.46 to 38.98), 34.04% (95% CI, 19.98 to 48.11), 46.15% (95% CI, 14.8 to 77.51), and 46.15% (95% CI, 14.8 to 77.51), respectively.
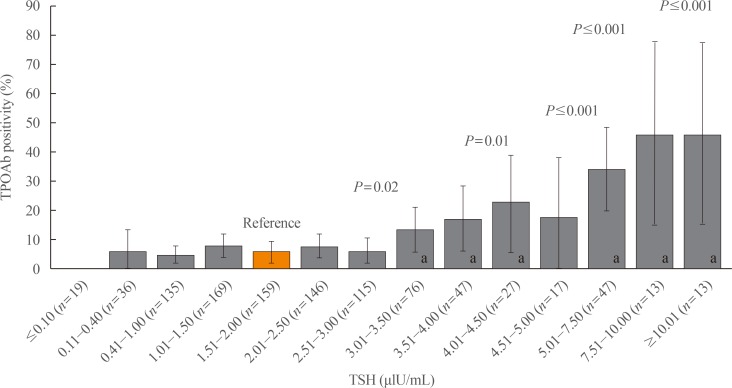
Table 1
Characteristics of the Study Population




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download