Abstract
Epidermal cysts are common benign subcutaneous lesions that occur in or on the skin. It is not very difficult to diagnose subcutaneous epidermal cysts using ultrasound imaging because they exhibit typical sonographic features. However, the differential diagnosis can be confused when epidermal cysts are found in unusual sites. The authors report a case involving a 4-year-old girl who presented with an intramuscular epidermal cyst in the gluteus maximus muscle. Magnetic resonance imaging revealed characteristic internal features of the epidermal cyst, despite being in an uncommon site, and was very useful in the preoperative diagnosis.
Epidermal cysts are common subcutaneous lesions and generally involve hair-bearing areas of the body including the scalp, face, neck, trunk, extremities, and scrotum (12). Pathologically, epidermal cysts are benign, confined by a wall of stratified squamous epithelial cells, and filled with keratin debris (3). They are also known as epidermal inclusion cysts, infundibular cysts, and epidermoid cysts (2). Epidermal cysts that occur in unusual sites have been reported in the literature as intraosseous, presacral, or splenic epidermal cysts (145).
However, an intramuscular epidermal cyst is extremely rare. The present article documents a case of a ruptured epidermal cyst that developed in the gluteus maximus muscle without skin involvement, and describes differential MRI features supporting the diagnosis of an epidermal cyst.
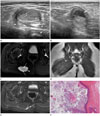
A 4-year-old girl presented with an incidentally detected painful and palpable mass in the right buttock. Physical examination revealed a firm movable mass at the inferomedial aspect of the buttock, which elicited tenderness during palpation. Cutaneous abnormalities, including hyperpigmentation, dermal sinus, or erythema of the skin, were not observed. She and her parents reported no history of trauma or previous surgery. Ultrasound imaging revealed a well-defined ovoid hyperechoic mass within the gluteus maximus muscle, measuring approximately 2.4 × 1.4 × 1.8 cm in size (Fig. 1A). The mass contained hyperechoic strands, with posterior acoustic enhancement, and did not exhibit internal hypervascularity on Doppler ultrasound.
Differential diagnoses included myxoma, intramuscular lipoma, organizing hematoma, thrombosed hemangioma, and injection granuloma. However, she had no apparent history of injection in the same point of the buttock, and the site of the mass appeared to be too medial to be caused by an injection into a muscle. The absence of internal vascularity reduced the possibility of a diagnosis of hemangioma. Additionally, the mass had neither calcifications nor an irregular margin to support a diagnosis of injection granuloma. Follow-up ultrasound was recommended.
Ten months later, the patient revisited the clinic complaining of tenderness in her buttock. Follow-up ultrasound revealed that the mass increased in size, now measuring 3 × 1.8 × 2.4 cm (Fig. 1B). The mass exhibited focal bulging and extended into the subcutaneous tissue layer. In addition, the anechoic cystic component of the mass at the subcutaneous extension site was noted. The possible diagnosis was intramuscular hemangioma with internal hemorrhage. However, it was important to exclude other intramuscular soft tissue tumors considering that the mass increased in size and exhibited altered characteristics on ultrasound.
She underwent MRI for further evaluation, which revealed a cystic lesion measuring 3.3 × 1.7 × 2.2 cm within the right gluteus maximus muscle. The lesion exhibited bright, high signal intensity on T2-weighted image and slightly high signal intensity on T1-weighted imaging (Fig. 1C, D). The mass included debris of slightly low signal serpentine foci on T2-weighted image with a high background signal. In particular, a tract with bright, high signal intensity on T2-weighted image similar to the material extending from the mass to the presacral space was noted. Additionally, focal budding and a portion of a superficial mass, which were not covered by the muscle layer and extended into the subcutaneous tissue layer due to cyst rupture, were apparent. After contrast administration, the lesion exhibited peripheral thin rim enhancement with focal irregular soft tissue enhancement near the mass (Fig. 1E). Based on MRI features of a well-defined cystic mass with internal debris, the primary differential diagnosis was a ruptured epidermal cyst. The cyst rupture may have resulted from its location, which was at the level of the ischial tuberosity, and repetitive irritation. Other differential diagnoses included myxoma or lymphangioma with internal hemorrhage.
The mass was subsequently excised with the patient under general anesthesia. The mass was well-defined and yellow-pink in color, and exhibited focal adhesion to the soft tissue at the ruptured portion. The mass was a ruptured cyst containing dirty sebaceous material. Hematoxylin and eosin stained microscopic sections revealed that it was a slightly atrophic squamous epithelial-lining cyst filled with multiple layers of keratin (Fig. 1F). Based on these findings, the patient was diagnosed with a ruptured epidermal cyst.
The majority of epidermal cysts are limited to the skin and subcutaneous tissue layer, and are the result of proliferation of epidermal cells within the dermal space. The etiology of epidermal cysts is unclear; however, several mechanisms have been proposed. Cysts in the skull or presacral space are believed to develop from remnant ectodermal tissues misplaced during embryogenesis. Other possible causes of epidermal cysts include occlusion of the pilosebaceous unit, or traumatic or surgical implantation of epithelial elements within the dermis (23). Invasion of surrounding structures by an epidermal cyst is uncommon. A notable exceptional case of an intraosseous epidermal cyst in the phalanx has been reported (4). However, to the best of our knowledge, only one case of intramuscular epidermal cyst-entirely confined to the muscle layer-has been reported in the English literature (6). That study described the cyst in the sternomastoid muscle of the neck. The patient previously sustained a severe injury in the same area with a heavy pointed rod. Unfortunately, the report did not provide any imaging evaluations. Additionally, two other case reports of giant epidermal cysts arising in the sole of the foot and buttock revealed intramuscular extension of the cysts into the interosseous and gluteus maximus muscles, respectively (78). However, they had a cystic portion connected to the skin, which explains the traumatic epidermal cell implantation theory into the dermis and, consequently, intramuscular extension.
In the present case, the cyst was mainly within the gluteus maximus muscle, with focal extension into the subcutaneous tissue through the cyst rupture site. Nevertheless, the cyst demonstrated no cystic portion connected with the dermis and skin layers, whereas a tract-like cystic portion was suspected of penetrating the presacral space in preoperative MRI review after performing the operation. Therefore, differential imaging diagnosis in the present case was highly confusing, and the mechanisms that led to the occurrence of the epidermal cyst remain unclear, despite the absence of trauma history or misplaced remnant ectodermal tissues similar to presacral epidermal cysts.
Various sonographic imaging features of epidermal cysts include a well-circumscribed, slightly echogenic mass, possibly demonstrating internal linear echogenic reflections or hypoechoic clefts, and the absence of vascularity on color Doppler imaging (9). On MRI, epidermal cysts exhibit fluid-like high signal on T2-weighted images and peripheral thin rim enhancement on gadolinium-enhanced images. Most ruptured cysts have septa, exhibit thick and irregular rim enhancement, and are accompanied by a fuzzy enhancement in surrounding subcutaneous tissue (910). In particular, an epidermal cyst is more likely than other fluid cysts when T2-weighted imaging demonstrates variable low-signal serpentine components within the mass, which reflects slightly viscous, grease-like content (10). The signal on MRI can change depending on the chemical composition of cholesterol and keratin. In our case, the mass also exhibited this characteristic low-signal debris on T2-weighted MRI, and the finding was suggestive of an epidermal cyst.
In conclusion, intramuscular epidermal cysts are extremely rare. However, an epidermal cyst should be considered in the differential diagnosis even if it is found in an unusual site when sonographic and MRI reveal characteristic features suggesting an epidermal cyst.
Figures and Tables
Fig. 1
Intramuscular Epidermal Cyst of the buttock in a 4-year-old girl presented with a palpable mass in the right buttock.
A. Ultrasound imaging revealing an ovoid hyperechoic mass with internal hyperechoic strands (short arrows) and posterior acoustic enhancement (arrowheads) in the gluteus maximus muscle on transverse scan. Acoustic shadowing by the ischial tuberosity (long arrow) is noted.
B. Ten months later, a follow-up ultrasound reveals an increase in the size of the mass, with extension to the subcutaneous tissue. The anechoic cystic change is indicated at a focal bulging portion to the subcutaneous layer (arrow).
C. Axial T2-weighted magnetic resonance image revealing a bright, hyperintense cystic mass in the right gluteus maximus muscle. The cyst includes internal hypointense debris (arrows) and the appearance of focal budding (arrowhead).
D. Coronal T1-weighted magnetic resonance image revealing a slightly high-intensity signal of the mass at the inferomedial aspect of the buttock (arrow).
E. Gadolinium-enhanced fat-suppressed T1-weighted image revealing peripheral rim enhancement and focal adjacent soft tissue enhancement (arrow).
F. Hematoxylin and eosin stained microscopic section revealing several layers of slightly atrophic squamous epithelial-lining cyst (black arrows) filled with horny materials (arrowheads). Pathological findings were consistent with epidermal cyst (× 200).
Acknowledgments
This study was written as part of Konkuk University's research support program for its faculty on sabbatical leave in 2015.
References
1. Yang DM, Yoon MH, Kim HS, Oh YH, Ha SY, Oh JH, et al. Presacral epidermoid cyst: imaging findings with histopathologic correlation. Abdom Imaging. 2001; 26:79–82.

2. Lever WH, Lever GS. Tumors and cysts of epidermis. In : Elder D, editor. Histopathology of the skin. 8th ed. Philadelphia, JB: Lippincott-Raven;1997. p. 685–746.
3. Elder D, Elenitsas R, Jaworsky C, Johnson JB. Lever's histopathology of the skin. 8th ed. Philadelphia: Lippincott-Raven;1997. p. 695–721.
4. Patel K, Bhuiya T, Chen S, Kenan S, Kahn L. Epidermal inclusion cyst of phalanx: a case report and review of the literature. Skeletal Radiol. 2006; 35:861–863.

5. Garg M, Kataria SP, Sethi D, Mathur SK. Epidermoid cyst of spleen mimicking splenic lymphangioma. Adv Biomed Res. 2013; 2:49.

6. Chatterjee PK, Chandra AB, Dastidar N. Epidermal cyst in sternomastoid muscle simulating a malignant growth. Indian J Otolaryngol Head Neck Surg. 1976; 28:86–87.
7. Ozawa T, Harada T, Ishii M. Giant epidermal cyst extending from sole to dorsum of the foot by penetrating the interosseous muscles. J Dermatol. 2008; 35:25–28.

8. Low SF, Sridharan R, Ngiu CS. Giant epidermal cyst with intramuscular extension: a rare occurrence. BMJ Case Rep. 2015; 2015:bcr2013202534.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download