Abstract
Purpose
To evaluate value of image subtraction for identifying hepatocellular carcinoma (HCC) capsule on gadoxetic acid-enhanced MR images.
Materials and Methods
This study involved 108 patients at risk of HCC preoperatively examined using gadoxetic acid-enhanced MRI with hepatic resection between May 2015 and February 2017. We evaluated qualities of subtraction images and presence of capsular appearance on portal venous or transitional phases conventional and subtraction images. We assessed effect of capsular appearance on subtraction images on HCC.
Results
After excluding 1 patient who had treated by transarterial chemoembolization prior to surgery and 33 patients with unsatisfactory subtraction image qualities, 82 focal hepatic lesions (73 HCC, 5 non-HCC malignancies, and 4 benign) from 74 patients were analyzed. Regarding detection of capsules, sensitivity, accuracy, and area under the receiver operating characteristic curve (AUC) on subtraction images were significantly higher than those on conventional images (95.4%, 89.0%, and 0.80, respectively; p < 0.001), though specificities were same (64.7%). For diagnosis of HCC, sensitivity, accuracy, and AUC on subtraction images were significantly higher than on conventional images (82.2%, 79.3%, and 0.69, respectively; p = 0.011), though specificities were identical (55.6%).
Hepatocellular carcinoma (HCC) is the fifth most common cancer globally (1). HCC capsules that develop during hepatocarcinogenesis are characteristic of nodular HCC (2). These capsules consist of peritumoral fibrosis and prominent sinusoids, and are observed in about 70% of nodular HCCs (34). Therefore, the presence and identification of capsules are important for the non-invasive diagnosis of HCC. The United Network for Organ Sharing (UNOS)-Organ procurement transplant network (OPTN) system and the Liver Imaging Reporting and Data System (LI-RADS) include an encapsulated appearance as a feature of HCC (56). Furthermore, the existence of an intact capsule is associated with better survival rates and lower recurrence rates after resection or ablative therapy (78).
Contrast-enhanced MRI using dynamic contrast enhanced T1-weighted gradient echo sequences is the most reliable technique for detecting HCC capsules (49). Extracellular and hepatobiliary gadolinium-based contrast agents (gadoxetic acid; Primovist or Eovist; Bayer Healthcare, Berlin, Germany) are commonly used for liver MRI. However, since the pharmacokinetic and pharmacodynamics of these two contrast agents differ, they have different effects on capsule appearances. Some investigators have reported capsular appearance of HCC is less frequently observed and less discernable on gadoxetic acid-enhanced MR images due to relatively high backgrounds of hepatic parenchymal enhancement (101112).
Subtraction of unenhanced images from gadolinium-enhanced images has been reported to enable better characterization of the enhancement patterns of focal liver lesions and to maximize lesion visualization (131415). In the present study, we evaluated the usefulness of subtraction images generated from portal or transitional phase (TP) images with respect to the detection of HCC capsule on gadoxetic acid-enhanced MR images.
Our Institutional Review Board approved this retrospective study and waived the requirement for informed consent due to its retrospective nature (IRB No. CR316128). From May 2015 to February 2017, a total of 108 consecutive patients at risk of HCC (91 with liver cirrhosis and 17 with chronic viral hepatitis) underwent preoperative gadoxetic acid-enhanced liver MRI and hepatic resection within 3 months. One patient who had been treated by transarterial chemoembolization prior to surgery was excluded. The remaining 107 patients (71 men, 36 women; mean age, 64 years; range, 45–88 years) had 122 focal hepatic lesions, that is; 108 HCCs, 6 cholangiocarcinomas, 2 combined HCC and cholangiocarcinoma, 1 metastatic adenocarcinoma, 1 high grade dysplastic nodule, 1 regenerative nodule, 2 focal nodular hyperplasia-like nodules, and one intrahepatic splenosis. Eleven patients had multiple HCCs (9 had two HCCs, one had three HCCs, and one had four HCCs) and one patient had one HCC with one combined HCC and cholangiocarcinoma.
MRI was performed using a 3.0-Tesla system (Magnetom Skyra; Siemens, Erlangen, Germany) equipped with an abdominal 64-channel surface coil. Localizer images were obtained in the supine position, and spectrally fat-suppressed breath-hold T2-weighted turbo spin echo images [repetition time (TR) = 920 ms, echo time (TE) = 106 ms, flip angle = 120°, echo train length = 94, slice thickness = 5 mm] were obtained in the axial plane. After obtaining a double-echo chemical shift gradient-echo sequence [TR = 4.46 ms, first-echo TE = 1.42 ms (opposed-phase), second-echo TE = 2.85 ms (in-phase), flip angle = 9°], dynamic studies were performed using a three-dimensional gradient echo sequence (VIBE; Siemens) with ultrafast image reconstruction using parallel imaging algorithms (GRAPPA factor = 2) in the axial plane (TR = 4.2 ms, TE = 2.0 ms, flip angle = 13°, matrix = 352 × 192, field of view = 345 × 380 mm, slice thickness = 2.2 mm, slice spacing = 0 mm, slices = 96) during a 20-second breath-holding period. For dynamic studies, gadoxetic acid disodium (0.1 mL/kg) (Primovist; Bayer Healthcare, Berlin, Germany) was administered at 2 mL/s with a power injector as a rapid bolus immediately followed by a 30 mL saline flush. Images were acquired before and after the intravenous injection of gadoxetic acid disodium. The acquisition delay for the arterial phase was usually 20–30 seconds and was determined using a bolus-tracking technique or a test-bolus injection technique. Dynamic images were obtained 30, 60 (portal venous phase, PVP), 180 (TP) and 300 seconds after injection. Hepatobiliary phase images were obtained 20 minutes after contrast injection. Unenhanced images were electronically subtracted from arterial, portal venous, and TPs by an MRI technologist using the system's commercially available software. All scans were forwarded to a picture archiving and communication system.
One board-certified abdominal radiologist with 4 years of experience, who was not involved in the subsequent image analysis, prepared a list indicating the size and locations of histopathology-proven lesions to be analyzed without indicating any imaging features.
Two board-certified abdominal radiologists (with 11 and 18 years of experience of liver MRI, respectively) blinded to final diagnoses, independently analyzed the MR images. During the image interpretation, the readers referred to the list indicating the anatomic locations and sizes of target lesions to ensure correct identification. After completing the independent review, two readers resolved discordances by discussion and achieved consensus in all cases. Interobserver agreement was assessed using kappa statistics.
The two readers also analyzed the image qualities of subtraction images using the following 5-point scale (16): 1) denoted overall nondiagnostic image quality, 2) denoted severe subtraction artifacts (maximum thickness of subtraction bands of > 5 mm or more than two-thirds of either liver margins or vascular markings ill-defined), 3) denoted moderate subtraction artifacts (maximum thickness of subtraction bands of 2.5 mm, or between one-third and two-thirds of either the liver margins or vascular markings ill defined), 4) denoted good overall image quality with minimal artifacts (maximum thickness of subtraction bands < 2 mm, or less than one-third of either the liver margins or vascular markings ill-defined), and 5) denoted perfect overall subtraction quality without any artifacts. Grades 4 and 5 were considered to indicate satisfactory image quality for subtraction images. Lesions with unsatisfactory image qualities were excluded from further evaluation.
The readers determined the presence or absence of a capsular appearance (a peripheral rim of smooth hyperenhancement) on PVP and/or TP, and LI-RADS categories: (LR-1; definitely benign, LR-2; probably benign, LR-3; indeterminate, LR-4; probably HCC, LR-5; definitely HCC, LR-5V; tumor in vein, and LR-M; probably malignant, but not specific for HCC) for each of the 82 focal hepatic lesions. Two weeks later (to avoid a recall bias), the readers determined the presence or absence of a capsule on subtraction images of PVP and/or TP, and LI-RADS categories of each of the hepatic lesions.
The detection rates of an capsular appearance by visual assessment of PVP and/or TP and using subtraction images of PVP and/or TP were compared using receiver operating characteristic (ROC) curve analysis.
To assess the clinical effects of subtraction images for the diagnosis of HCC using the LI-RADS diagnostic algorithm, we compared the sensitivities, specificities, and accuracies of LR-5 or LR-5V for HCC between the following two situations: 1) when conventionally defined capsular appearance was included as a major feature, and 2) when capsular appearance defined on subtracted images was included as a major feature. Pathological results were used as reference standards.
Interobserver agreement between imaging findings (before consensus) was determined using weighted kappa statistics. Levels of agreement were defined as follows: poor, κ ≤ 0.20; fair, κ = 0.21–0.40; moderate, κ = 0.41–0.60; good, κ = 0.61–0.80; excellent, κ = 0.81–1.00. Two-sided p values of less than 0.05 were considered to indicate statistical significance. The analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA), MedCalc software (MedCalc Software, Ostend, Belgium), and R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Thirty-three of the 107 patients (30.8%) showed a misregistration artifact (Grade 1, one patient; Grade 2, five patients; and Grade 3, 27 patients), and the images concerned were considered unsatisfactory.
After excluding the 33 patients with unsatisfactory subtraction images, we finally analyzed 74 patients with 82 focal hepatic lesions (73 HCCs, 2 cholangiocarcinomas, 2 combined HCC and cholangiocarcinoma, 1 metastatic adenocarcinoma, 1 high grade dysplastic nodule, 1 regenerative nodule, and 2 focal nodular hyperplasia-like nodules). The baseline characteristics of the 74 patients and 82 focal hepatic lesions are summarized in Table 1.
Pathologically, 63 (86.3%) of the 73 HCCs and 2 (40%) of the 5 other malignancies (1 combined HCC and cholangiocarcinoma, 1 cholangiocarcinoma) had a fibrous capsule. None of 4 benign lesions had a capsule.
Imaging evaluations of capsules revealed good agreement between the two reviewers on conventional (κ = 0.68) and subtraction images (κ = 0.72). Diagnostic performances of hepatocellular capsules on conventional images and subtraction images are summarized in Table 2. The sensitivity and accuracy of detection of HCC on subtraction images were higher than those on conventional images, but specificity did not differ. In addition, area under ROC curve (AUC) was significantly higher for subtraction images than conventional images (p < 0.001) (Fig. 1).
When focal hepatic lesions were categorized using subtraction images, the AUC of LR-5 or LR-5V for diagnosing HCC were significantly higher than the AUC of conventional images (0.65 and 0.69, respectively) (p = 0.011) (Table 3).
In the present study, it was found subtraction images detected the capsular appearance of HCC more sensitively than conventional images (95.4% and 73.9%, respectively) without loss of specificity (64.7% and 64.7%, respectively), and that the diagnostic performances of LR-5 and LR-5V, including capsular appearance as a major feature, using subtraction images was significantly higher than that of conventional images (p = 0.011).
Histologically, HCC capsules are composed of two layers; an inner layer rich in fibrous tissue and an outer, water-rich layer with portal venules (or sinusoids) and newly-formed bile ducts (34). However, imaging does not always show a true fibrous capsule; instead, a pseudocapsule consisting of peritumoral sinusoids and/or fibrosis may be evident (417). This capsular appearance, be it a true fibrous capsule or pseudocapsule, is one of the specific findings of HCC, because benign nodules and non-HCC malignancies usually do not manifest a capsular appearance (1819202122).
Fibrous capsules and pseudocapsules have similar appearances on dynamic MR images, and appear as enhancing rims on PVP or TP images due to retention of contrast agent within prominent peritumoral sinusoids and/or fibrosis (9). Approximately 50% of gadoxetic acid is uptaken by hepatocytes, and this uptake starts with first pass through the liver; in fact, it has been reported to become visible as early as 90 sec after injection (23). Furthermore, this rapid uptake by hepatocytes also explains the rapid removal of gadoxetic acid from capsule sinusoids, and therefore, capsules are faint and not well identified on PVP or TP images.
However, according to our results, the conspicuity of a capsule or pseudocapsule is improved on subtraction images generated from PVP or TP images. One possible explanation for this result might be that subtraction images more prominently represent subtle differences in contrast enhancement than conventional images, which in turn suggests subtraction images better depict capsular appearance than conventional images.
Although some investigators have found that the capsular appearance does not increase diagnostic accuracy for HCC (20), others have asserted that a visualization of a capsule is valuable, as it permits diagnosis of HCC without a definite washout appearance (624). Furthermore, capsular appearance is included as a major imaging feature in the LI-RADS diagnostic algorithm and plays an important role in the diagnosis of HCC. This algorithm considers a hyperintense capsular appearance during PVP or TP as a major diagnostic feature of non-invasive HCC (6), and diagnostic performances of LR-5 and LR-5V using capsular appearance on subtraction images for HCC, are higher than that of conventional imaging. Our results imply that subtraction images can perform an important compensatory role in terms of evaluating capsular appearance when hepatobiliary contrast agents are used. According to recent literature, in patients at risk of developing HCC, a combination of arterial phase hyperenhancement plus washout or a capsular appearance has near 100% specificity for HCC (10). However, in the present study, the specificities of LR-5 and LR-5V for HCC were poor (55.6%), which appeared to be related to several false positive results attributed to the presence of a pseudocapsule.
Previous studies have reported the success of the subtraction technique depends on the degree of misregistration artifact between non-enhanced and enhanced source images (1314). The assessment of subtraction image quality is important because false positive diagnoses can be made for capsules when misregistration occurs. In our study, subtraction image quality was unsatisfactory for 33 of the 107 patients (30.8%) showed (Grade 1 in one patient; Grade 2 in five patients; and Grade 3 in 27 patients). Although image quality was unsatisfactory in many patients, the accuracy of capsule detection was high when subtraction images were of Grade 4 quality or better. There were 6 false positive cases for capsular appearance on subtraction images, and all of these cases produced a false positive result on conventional images. Therefore, these 6 false positive cases were considered to be due to pseudocapsules rather than to misregistration of subtraction images. Nevertheless, unavoidable slice misregistration is the main pitfall of subtraction techniques. Reliable error-correction processing methods for subtraction imaging are needed (14).
This study has several limitations that warrant consideration. First, the sample size was too small to permit definitive conclusions, although all lesions including were confirmed histopathologically. The study was powered to detect a > 10% difference in capsule detection rates with an α of 0.05. Based on the actual sample size, the study has a statistical power of 76.0%. Further studies on larger populations are needed to confirm the value of subtraction images for HCC capsule detection. Second, the retrospective nature of the present study inherently introduces the possibility of patient selection bias. Finally, a 2-week interval may have been insufficient to avoid recall bias.
In summary, we conclude subtraction images from portal or TP images may be helpful for detecting HCC tumor capsules on gadoxetic acid-enhanced MR images in at-risk patients.
Figures and Tables
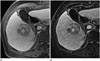 | Fig. 1Case of a 73-year-old woman with hepatocellular carcinoma encased by a histopathologically confirmed fibrous capsule in the right liver
A. A conventional capsular appearance is not well visualized in the conventional TP image.
B. However, a smooth hyperintense rim is readily detected on the subtracted TP image.
TP = transitional phase
|
Table 1
Clinical Characteristics of 74 Patients at Risk of HCC

Table 2
Comparison of the Diagnostic Performances of Conventional and Subtraction Images for the Detection of HCC on Gadoxetic Acid-Enhanced MR Images

Table 3
Comparison of the Diagnostic Performances of LR-5 or LR-5V for HCC

References
1. El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003; 139:817–823.

3. Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992; 183:819–825.

4. Ishigami K, Yoshimitsu K, Nishihara Y, Irie H, Asayama Y, Tajima T, et al. Hepatocellular carcinoma with a pseudocapsule on gadolinium-enhanced MR images: correlation with histopathologic findings. Radiology. 2009; 250:435–443.

5. Organ Procurement and Transplantation Network. OPTN/UNOS policy 9.3.G.iv. Accessed Mar 16, 2016. Available at: http://optn.transplant.hrsa.gov/ContentDocuments/OPTN_Policies.pdf-nameddest=Policy_09. Published Jan 1, 2015.
6. Liver Reporting and Data System, version 2013.1. American College of Radiology;Accessed Mar 16, 2016. Available at: http://www.acr.org/Quality-Safety/Resources/LIRADS/.
7. Miraglia R, Pietrosi G, Maruzzelli L, Petridis I, Caruso S, Marrone G, et al. Predictive factors of tumor response to trans-catheter treatment in cirrhotic patients with hepatocellular carcinoma: a multivariate analysis of pre-treatment findings. World J Gastroenterol. 2007; 13:6022–6026.

8. Ng IO, Lai EC, Ng MM, Fan ST. Tumor encapsulation in hepatocellular carcinoma. A pathologic study of 189 cases. Cancer. 1992; 70:45–49.

9. Grazioli L, Olivetti L, Fugazzola C, Benetti A, Stanga C, Dettori E, et al. The pseudocapsule in hepatocellular carcinoma: correlation between dynamic MR imaging and pathology. Eur Radiol. 1999; 9:62–67.

10. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014; 273:30–50.

11. Dioguardi Burgio M, Picone D, Cabibbo G, Midiri M, Lagalla R, Brancatelli G. MR-imaging features of hepatocellular carcinoma capsule appearance in cirrhotic liver: comparison of gadoxetic acid and gadobenate dimeglumine. Abdom Radiol (NY). 2016; 41:1546–1554.

12. Hope TA, Fowler KJ, Sirlin CB, Costa EA, Yee J, Yeh BM, et al. Hepatobiliary agents and their role in LI-RADS. Abdom Imaging. 2015; 40:613–625.

13. Yu JS, Kim YH, Rofsky NM. Dynamic subtraction magnetic resonance imaging of cirrhotic liver: assessment of high signal intensity lesions on nonenhanced T1-weighted images. J Comput Assist Tomogr. 2005; 29:51–58.
14. Yu JS, Rofsky NM. Dynamic subtraction MR imaging of the liver: advantages and pitfalls. AJR Am J Roentgenol. 2003; 180:1351–1357.

15. Seçil M, Obuz F, Altay C, Gencel O, Iğci E, Sağol O, et al. The role of dynamic subtraction MRI in detection of hepatocellular carcinoma. Diagn Interv Radiol. 2008; 14:200–204.
16. Sundarakumar DK, Wilson GJ, Osman SF, Zaidi SF, Maki JH. Evaluation of image registration in subtracted 3D dynamic contrast-enhanced MRI of treated hepatocellular carcinoma. AJR Am J Roentgenol. 2015; 204:287–296.

17. Cho ES, Choi JY. MRI features of hepatocellular carcinoma related to biologic behavior. Korean J Radiol. 2015; 16:449–464.

18. Asayama Y, Nishie A, Ishigami K, Ushijima Y, Takayama Y, Fujita N, et al. Distinguishing intrahepatic cholangiocarcinoma from poorly differentiated hepatocellular carcinoma using precontrast and gadoxetic acid-enhanced MRI. Diagn Interv Radiol. 2015; 21:96–104.

19. Khan AS, Hussain HK, Johnson TD, Weadock WJ, Pelletier SJ, Marrero JA. Value of delayed hypointensity and delayed enhancing rim in magnetic resonance imaging diagnosis of small hepatocellular carcinoma in the cirrhotic liver. J Magn Reson Imaging. 2010; 32:360–366.

20. Park HJ, Jang KM, Kang TW, Song KD, Kim SH, Kim YK, et al. Identification of imaging predictors discriminating different primary liver tumours in patients with chronic liver disease on gadoxetic acid-enhanced MRI: a classification tree analysis. Eur Radiol. 2016; 26:3102–3111.

21. Rimola J, Forner A, Tremosini S, Reig M, Vilana R, Bianchi L, et al. Non-invasive diagnosis of hepatocellular carcinoma ≤ 2 cm in cirrhosis. Diagnostic accuracy assessing fat, capsule and signal intensity at dynamic MRI. J Hepatol. 2012; 56:1317–1323.
22. Suh YJ, Kim MJ, Choi JY, Park YN, Park MS, Kim KW. Differentiation of hepatic hyperintense lesions seen on gadoxetic acid-enhanced hepatobiliary phase MRI. AJR Am J Roentgenol. 2011; 197:W44–W52.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download