Abstract
Purpose
The purpose of this study was to evaluate the effectiveness of thoracic paravertebral block (TPVB) for management of pain during and after percutaneous radiofrequency ablation (RFA) of hepatic tumor.
Materials and Methods
All patients were divided into non-TPVB (4 patients, 4 sessions of RFA for 4 tumors) and TPVB group (5 patients, 7 sessions of RFAs for 7 tumors). Ultrasound (US)-guided TPVB was performed at T7 level. The 15 mL of 0.375% ropivacaine was injected into right paravertebral space before RFA. If patients complained pain and asked analgesics or experienced pain with verbal numerical rating scale (VNRS) of more than 4, fentanyl 25 µg (up to 100 µg), pethidine 25 mg, and midazolam 0.05 mg/kg (up to 5 mg) were sequentially given intravenously during RFA.
Radiofrequency ablation (RFA) is accepted as an effective and safe technique for local treatment of primary and secondary hepatic tumor (1234). However, RFA of hepatic tumor can cause several side effects like post-ablation syndrome, pleural effusion, perihepatic fluid or blood collections (5). The pain is also classified into side effect of RFA according to the standardization of terminology and reporting criteria of image-guided tumor ablation (5). Moderate to severe pain during RFA can eliminate patient's cooperation and provoke unexpected action, especially in patients sensitive to pain, and may result in early interruption of RFA or increasing risk of complications. Therefore, RFA is usually performed under conscious sedation or general anesthesia (6789101112). However, even with appropriate conscious sedation techniques, many patients experience pain during RFA procedure (56). After RFA, many patients experience grade 1–2 pain (the Common Toxicity Criteria of the National Cancer Institute for reporting pain) for several days, occasionally lasting for 1–2 weeks following an ablation procedure (5).
Thoracic paravertebral block (TPVB) has been often used for management of pain in unilateral surgical procedures such as thoracotomy, major breast surgery, cholecystectomy, renal surgery, and laparotomy (13).
The purpose of this study was to evaluate the effectiveness of ultrasound (US)-guided TPVB for management of pain during and after percutaneous RFA of hepatic tumor.
This study was approved by the Institutional Review Board of Presbyterian Medical Center (PMCIRB-005-001). Written informed consent was obtained from patients about the study during pre-anesthetic visitation. Nine patients underwent 11 sessions of US-guided percutaneous RFA for 11 hepatic tumors. Nine tumors of 8 patients met the following criteria for treatment with percutaneous RFA: a single nodular hepatocellular carcinoma (HCC) not greater than 5 cm in maximum diameter; up to three multinodular HCCs, with each tumor measuring up to 3 cm in maximum diameter; absence of portal venous thrombosis; Child-Pugh classification (CPC) A or B liver cirrhosis; a prothrombin time ratio > 50%; and a platelet count greater than 70000 cells/mm3 (6). The other one patient had two metastatic tumors with each tumor less than 3 cm in maximum diameter (Table 1).
Two metastatic carcinomas (in 1 patient) and one HCC (in 1 patient) were confirmed histopathologically by US-guided percutaneous needle biopsy. The remaining 8 tumors in 7 patients were considered to be HCC on the basis of 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of HCC (14). Two HCCs in 2 patients were recurred tumors in segment 2 and 3 after right posterior sectionectomy and right hemihepatectomy, respectively (Table 1).
All procedures were performed under continuous monitoring of electrocardiogram, pulse oximetry, and non-invasive blood pressure until the end of RFA.
All patients were randomly assigned to non-TPVB and TPVB group. The medication used before RFA in both group and the characteristics of patients and tumors of each group are summarized in Fig. 1 and Table 1, respectively.
Non-TPVB group consisted of 4 patients (four sessions of RFA for four tumors). All patients in this group received 0.5 mg of atropine and 25 mg of pethidine intramuscularly as premedication on ward. And all patients received 50 µg of fentanyl intravenously as premedication in operating room. After deciding insertion site and path of RF electrode with US, 1% lidocaine was infiltrated.
Seven sessions of RFAs for 7 tumors in 5 patients were performed after US-guided TPVBs. In this group, pethidine was not given on ward before RFA. After deciding insertion site and path of RF electrode with US, US-guided TPVB was performed with patients in lateral position with the side to be blocked uppermost. All US-guided TPVB were performed by one experienced anesthesiologist. The T7 transverse process was used as the landmark of the T7 paravertebral space and was determined by confirming the connection with the 7th rib on the ultrasonogram. The 7th rib was determined by counting up from 12th rib on the posterior or counting down from 2nd rib on the anterior. Transportable US equipment with a 50 mm linear 15-6 MHz probe (SonoSite M-Turbo™; SonoSite Inc., Bothell, WA, USA) and epidural Tuohy needle (22-gauge, 80 mm) were used. After surgical disinfection of both cervical-thoracic paravertebral areas, US-guided TPVB was performed at T7 level after skin infiltration with 1 mL of 1% lidocaine. Fifteen mL of 0.375% ropivacaine was injected into right paravertebral space before RFA. TPVB was confirmed appropriate spread of ropivacaine into paravertebral space by anterior displacement of the pleura on US image (Fig. 2). We evaluated cutaneous sensory block by cold test (Fig. 3), then performed RFA. In this group, lidocaine for anesthetizing inserting site and path of RF electrode was not used.
The medications used during RFA in both group are summarized in Fig. 1. During RFA, pain was measured with verbal numerical rating scale (VNRS) which using 11 point scale, with 0 (no pain) to 10 (worst possible pain). We defined pain frequency during RFA as the number of injection of analgesics with/without sedatives. During RFA, if patients asked analgesics or experienced pain with a VNSR of more than 4, fentanyl 25 µg (up to 100 µg), pethidine 25 mg, and midazolam 0.05 mg/kg (up to 5 mg) were sequentially given intravenously during RFA. (Fig. 1). The pain after RFA was measured by the period that analgesics were given and total dose of analgesics given after RFA. The information about analgesics used after RFA were achieved from medical records. Total dose of analgesics used before, during and after RFA were converted to equivalent dose of morphine given intravenously (intravenous morphine equivalence) (15, 16).
All percutaneous RFA were performed under US-guidance on inpatient basis by one interventional radiologist. We used internally cooled 17-gauge electrodes (Well point RF electrode; STARmed, Goyang, Korea) with 3 cm exposed metallic tip with a 500-KHz monopolar radiofrequency generator (Valleylab; Covidien, Mansfield, MA, USA) capable of producing 200 W. All ablations were performed routinely for 12 minutes for each tumor. Additional ablation was performed until securing safety margin. We cauterized the electrode path during retraction of the electrode to minimize bleeding after ablation. Multiple tumors were ablated each with interval of one week.
Contrast enhanced CT was performed immediately after ablation and evaluated whether the tumor was covered completely.
For determining tumor location, we drew the two tangential lines to both lateral wall of vertebral body on axial CT image (Fig. 4). On axial CT image showing the greatest tumor diameter, if more than 50% of tumor area was included between 2 lines, tumor location was defined as middle position. If less than 50% of tumor area was between 2 lines, location was decided as right or left according to side including more than 50% of tumor area. On follow up CT, if ablation zone most close to abdominal wall was between 2 lines, right to right line, and left to left line, insertion site of RF electrode was defined as middle portion, right, and left, respectively.
Because of limited study subjects, the data were presented as median and 25th, 75th percentile. We used Mann-Whitney U test or Fisher's exact test (gender) for comparison between TPVB and non-TPVB groups. SPSS 23.0 (IBM Corp., Armonk, NY, USA) were used for data presentation and analysis. p-value lower than 0.05 were considered statistically significant.
The study group consisted of 3 women and 6 men with a median age of 61.0 years (range, 43–78). Patients had alcoholic cirrhosis (n = 3), cirrhosis due to hepatitis B infection (n = 5), and history of radical cholecystectomy for gallbladder cancer (n = 1). Target masses consisted of HCC (n = 9) and metastatic carcinoma (n = 2) (Table 1). The location of tumor and insertion site of RF electrode were summarized in Table 1.
In non-TPVB group, there was intra-procedural pain in all sessions of RFA (100%) and median pain frequency per one session of RFA was median 3.75 (25th and 75th percentile were 3.3 and 4.0). Median VNRS score per one session of RFA was 6.0 (6.0, 6.4). In all cases, 100 µg of fentanyl and 25 mg of pethidine were given during RFA. In three patients of four patients, midazolam was given once intravenously (Tables 2, 3). The median value of intravenous morphine equivalence of analgesics given during RFA was 13.3 mg per one session of RFA.
In TPVB group, patients complained intra-procedural pain in 3 sessions of RFA (42.86%) and 25th and 75th pain frequency were 0.0 and 1.0. times per RFA. Median VNRS score was 0.0 (0.0, 4.0) per one session of RFA. Median 25 µg of fentanyl was given during one session of RFA. Pethidine and midazolam were not used in all case. The median intravenous morphine equivalence of analgesics given during RFA was 0.0 (0.0, 2.5) mg per one session of RFA. In this group, the patient with the most frequent pain (3 times) had one middle positioned tumor. The intravenous morphine equivalence of this patient was 7.5 mg. The other 2 patients with pain during RFA had the pain frequency of just one time and a right positioned tumor each. The intravenous morphine equivalence of these 2 patients was 2.5 mg each (Tables 2, 3).
In non-TPVB group, all patients complained post-RFA pain during median 7.5 days (25th, 75th percentile; 1.0, 19.3). In 1 patient, tramadol was given intravenously (intravenous morphine equivalence = 362 mg) for 21 days after RFA. In 1 patient, tramadol, pethidine, and oxycodone were given per oral or intravenously (intravenous morphine equivalence = 221 mg) during 14 days. Other 2 patients, tramadol was used intravenously (intravenous morphine equivalence = 4 mg) for 1 day after RFA (Table 2). The intravenous morphine equivalence of analgesics given during RFA was median 112.5 mg (4.0, 326.8) per one session of RFA.
The pain is classified into side effect of RFA according to the standardization of terminology and reporting criteria of image-guided tumor ablation (5). For analgesia and/or conscious sedation, many drugs like pethidine, fentanyl, midazolam, and pentazocine, etc. are being used before and during RFA (678910). However, even with appropriate analgesia and/or conscious sedation, most patients complain the pain during and after ablation procedure (56). Moderate to severe pain during RFA can eliminate patient's cooperation and provoke unexpected action, especially in patients sensitive to pain, and may result in failure of ablation procedure or complications. Also, the pain after RFA can prolong the duration of admission. After RFA, most patients complain grade 1–2 pain (the Common Toxicity Criteria of the National Cancer Institute for reporting pain) for one or two weeks, but occasionally lasting more weeks following RFA (5).
There were few reports about pain during and after RFA for hepatic tumor, as we know. Lee et al. (6) reported that tumor adjacent (< 2 cm) to the parietal peritoneum is an independent predictor of higher pain level during percutaneous RFA. Also they reported that severity of pain during RFA was correlated with several factors such as tumor size, previous treatment, multiplicity of RFA, and duration of RFA, and affect pain after RFA presented by supplemental narcotics during hospitalization. They used conscious sedation with the use of pethidine for RFA. Including this study, most studies performed RFA under conscious sedation and some studies used general anesthesia (67891012).
TPVB is the technique of injecting local anesthetic into the thoracic paravertebral space (TPVS), which contains spinal nerves and sympathetic trunk (17). TPVB produces unilateral, segmental, somatic, and sympathetic nerve blockade due to a direct effect of the local anesthetic on the somatic and sympathetic nerves in the TPVS and extension into the intercostal and epidural space (1718). TPVB is indicated for anesthesia and analgesia for unilateral surgical procedures in the chest and abdomen. Based on published data, the incidence of complications after TPVB is relatively low (2.6–5%) (19). These include vascular puncture (3.8%), hypotension (4.6%), pleural puncture (1.1%), and pneumothorax (0.5%) (19). The US-guided TPVB may decrease the incidence of complications such as vascular puncture, pleural puncture, and pneumothorax. Unlike with thoracic epidural anesthesia, hypotension is rare in normovolemic patients after TPVB because the sympathetic blockade is unilateral. Some studies (202122) reported that TPVB was safe and effective for anesthesia and analgesia during percutaneous RFA of hepatic tumor. Similarly, US-guided TPVB was very effective for control of pain during and after RFA in our study. Unlike those studies, we compared TPVB and conventional conscious sedation (non-TPVB). In non-TPVB group, intra-procedural pain occurred in 100% of session of ablation. On contrast, there was intra-procedural pain in 42.9% of session of ablation in TPVB group. There were statistically differences between TPVB and non-TPVB group in all aspects including pain frequency, pain severity (presented by VNRS), and intravenous morphine equivalence of analgesics during RFA, duration of pain and intravenous morphine equivalence of analgesics after RFA, and total intravenous morphine equivalence of analgesics (p < 0.05) (Table 3). During RFA, non-TPVB group complained about greater than moderate pain several times on every session. However the severity and frequency of pain were very little to none in TPVB group.
Chronic postsurgical pain (CPSP) is the consequence of acute postoperative pain. Predictive factors for CPSP can be patient specific or surgery specific. These factors can be subdivided into preoperative, intraoperative, and postoperative. The most relevant postoperative factor seems to be the severity of acute postoperative pain (2324). In our study, most promising result was that there was no pain after RFA in TPVB group. Two out of 4 patients from non-TPVB group had analgesic given since they suffered from severe pain after RFA for 14 days and 21 days each. None of the patients from 7 sessions (5 patients) of TPVB group complained about pain after RFA. Therefore TPVB may decrease the risk of CPSP after RFA of hepatic tumor.
Our study has a few limitations. The first limitation is very small sample size. Because of this small size of this study, our results should be interpreted with caution and need to be supported by another study with larger sample size. The second limitation is that we performed only right-sided TPVB and there was no tumor with left sided location and left sided insertion site. In TPVB group, 3 sessions (3 patients) of 7 sessions (5 patients) had pain during RFA, although frequency and severity was significantly lesser than non-TPVB group. Especially 1 patient had more frequent and more severe pain than other 2 patients. In this patient, the location of the tumor was middle position according to our criteria. Although there was another patient with middle positioned tumor without pain during RFA, we think that right-sided TPVB alone might have not been enough to control the visceral pain since the location of the tumor was middle position. Although we cannot make a hasty conclusion due to limited sample size and limited location of tumor, we think additional left-sided TPVB, i.e. both-sided TPVB, may have a promising effect on control pain during RFA of tumor with middle or left position. The third limitation is that we performed only single injection in TPVB. So, due to this single injection, additional opioid analgesics and/or sedatives were needed according to the degree of pain and/or patient's demand. If we perform TPVB with catheterization, additional injection of local anesthetics can be done as needed. Also, we expect the catheterization may make pain management without opioid and post-procedural pain management possible. The fourth limitation is that we did not have long term follow up for CPSP. There are no reports about CPSP after RFA, as we know. Although TPVB reduced the severity of acute postoperative pain after RFA of hepatic tumor in our study, we did not study whether RFA of hepatic tumor can be surgery specific predictive factor for CPSP and do not know whether TPVB have prophylactic effect against CPSP after RFA of hepatic tumor. We think a well-planned prospective study is required to correlate RFA of hepatic tumor with CPSP.
In conclusion, US-guided TPVB may be an effective and safe anesthetic method for decreasing or eliminating pain during and after RFA for hepatic tumor. And US-guided TPVB may be helpful in decreasing opioid consumption during and after RFA for hepatic tumor.
Figures and Tables
 | Fig. 1The flowchart of premedication and pain management during RFA. If patients asked for analgesics or experienced pain with a verbal numerical rating scale score of more than 4, fentanyl 25 µg (up to 100 µg), pethidine 25 mg, and midazolam 0.05 mg/kg (up to 5 mg) were given intravenously during RFAHCL = hydrochloride, IM = intramuscularly, IV = intravenously, RFA = radiofrequency ablation
|
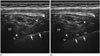 | Fig. 2Ultrasound image of the thoracic paravertebral space before (A) and after (B) administration of the local anesthetic. After administration of the local anesthetic, the pleura (arrows) was displaced anteriorly (arrowhead, needle and tip of needle; aserisk, thoracic paravertebral space).LA = local anesthetic, TP = transverse process
|
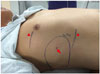 | Fig. 3Area of sensory loss following thoracic paravertebral block (arrow, needle insertion site of Radiofrequency ablation; arrowheads, upper and lower margin of sensory loss). |
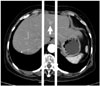 | Fig. 4Location of tumor is marked (arrow) as either right, middle, and left with reference to the vertebral column. |
Table 1
The Characteristics of Patients and Tumors

Table 2
Pain and Analgesics during and after RFA

Table 3
Comparison between TPVB and Non-TPVB Groups

References
1. Salhab M, Canelo R. An overview of evidence-based management of hepatocellular carcinoma: a meta-analysis. J Cancer Res Ther. 2011; 7:463–475.

2. Wu YZ, Li B, Wang T, Wang SJ, Zhou YM. Radiofrequency ablation vs hepatic resection for solitary colorectal liver metastasis: a meta-analysis. World J Gastroenterol. 2011; 17:4143–4148.

3. Carrafiello G, Laganà D, Cotta E, Mangini M, Fontana F, Bandiera F, et al. Radiofrequency ablation of intrahepatic cholangiocarcinoma: preliminary experience. Cardiovasc Intervent Radiol. 2010; 33:835–839.
4. Yang W, Chen MH, Yin SS, Yan K, Gao W, Wang YB, et al. Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early and late phase recurrence. AJR Am J Roentgenol. 2006; 186:5 Suppl. S275–S283.
5. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005; 235:728–739.

6. Lee S, Rhim H, Kim YS, Choi D, Lee WJ, Lim HK, et al. Percutaneous radiofrequency ablation of hepatocellular carcinomas: factors related to intraprocedural and postprocedural pain. AJR Am J Roentgenol. 2009; 192:1064–1070.

7. Park YR, Kim YS, Rhim H, Lim HK, Choi D, Lee WJ. Arterial enhancement of hepatocellular carcinoma before radiofrequency ablation as a predictor of postablation local tumor progression. AJR Am J Roentgenol. 2009; 193:757–763.

8. Takaki H, Yamakado K, Sakurai H, Nakatsuka A, Shiraki K, Iasaji S, et al. Radiofrequency ablation combined with chemoembolization: treatment of recurrent hepatocellular carcinomas after hepatectomy. AJR Am J Roentgenol. 2011; 197:488–494.

9. Kim YK, Kim CS, Chung GH, Han YM, Lee SY, Jin GY, et al. Radiofrequency ablation of hepatocellular carcinoma in patients with decompensated cirrhosis: evaluation of therapeutic efficacy and safety. AJR Am J Roentgenol. 2006; 186:5 Suppl. S261–S268.

10. Shibata T, Shibata T, Maetani Y, Isoda H, Hiraoka M. Radiofrequency ablation for small hepatocellular carcinoma: prosepctive comparison of internally cooled electrode and expandable electrode. Radiology. 2006; 238:346–353.

11. Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol. 2011; 2011:104685.

12. Chen MH, Yang W, Yan K, Gao W, Dai Y, Wang YB, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005; 11:6395–6401.

13. Richardson J, Lönnqvist PA. Thoracic paravertebral block. Br J Anaesth. 1998; 81:230–238.
14. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 KLCSG-NCC Korea practice guideline for the management of hepatocellular carcinoma. Gut Liver. 2015; 9:267–231.
15. Ripamonti C, Groff L, Brunelli C, Polastri D, Stavrakis A, De Conno F. Switching from morphine to oral methadone in treating cancer pain: what is the equianalgesic dose ratio. J Clin Oncol. 1998; 16:3216–3221.

16. Mercadante S, Casuccio A, Fulfaro F, Groff L, Boffi R, Villari P, et al. Switching from morphine to methadone to improve analgesia and tolerability in cancer patients: a prospective study. J Clin Oncol. 2001; 19:2898–2904.

18. Cheema SP, Ilsley D, Richardson J, Sabanathan S. A thermographic study of paravertebral analgesia. Anaesthesia. 1995; 50:118–121.

19. Lönnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995; 50:813–881.

20. Cheung Ning M, Karmakar MK. Right thoracic paravertebral anaesthesia for percutaneous radiofrequency ablation of liver tumours. Br J Radiol. 2011; 84:785–789.

21. Gazzera C, Fonio P, Faletti R, Dotto MC, Gobbi F, Donadio P, et al. Role of paravertebral block anaesthesia during percutaneous transhepatic thermoablation. Radiol Med. 2014; 119:549–557.

22. Piccioni F, Fumagalli L, Garbagnati F, Di Tolla G, Mazzaferro V, Langer M. Thoracic paravertebral anesthesia for percutaneous radiofrequency ablation of hepatic tumors. J Clin Anesth. 2014; 26:271–275.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download