Abstract
Objective
To investigate the progression risk of atypical squamous cells of undetermined significance (ASCUS) with different clinical managements.
Methods
Women with their first diagnosis of ASCUS cytology were retrieved from the national cervical cancer screening database and linked to the national health insurance research database to identify the management of these women. The incidences of developing cervical intraepithelial neoplasia grade 3 and invasive cervical cancer (CIN3+) were calculated, and the hazard ratios (HRs) were estimated using a Cox proportional hazards model. This study was approved by the Research Ethics Committee of the National Taiwan University Hospital and is registered at ClinicalTrials.gov (Identifier: NCT02063152).
Results
There were total 69,741 women included. Various management strategies including colposcopy, cervical biopsies and/or endocervical curettage, and cryotherapy, failed to reduce the risk of subsequent CIN3+ compared with repeat cervical smears. Loop electrosurgical excision procedure/conization significantly decreased risk of subsequent CIN3+ lesions (HR=0.22; 95% confidence interval [CI]=0.07–0.68; p=0.010). Women in their 40s–50s had an approximately 30% risk reduction compared to other age groups. Women with a previous screening history >5 years from the present ASCUS diagnosis were at increased risk for CIN3+ (HR=1.24; 95% CI=1.03–1.49; p=0.020).
Go to : 
References
1. Papanicolaou GN, Traut HF. Diagnosis of uterine cancer by the vaginal smear. New York, NY: Commonwealth Fund;1943.
2. Ayre JE. Selective cytologic smear for the diagnosis of cancer. Am J Obstet Gynecol. 1947; 53:609–17.
3. Gagnon F. Contribution to the study of the etiology and prevention of cancer of the cervix of the uterus. Am J Obstet Gynecol. 1950; 60:516–22.

4. Chen YY, You SL, Chen CA, Shih LY, Koong SL, Chao KY, et al. Effectiveness of national cervical cancer screening programme in Taiwan: 12-year experiences. Br J Cancer. 2009; 101:174–7.

5. Davey DD, Neal MH, Wilbur DC, Colgan TJ, Styer PE, Mody DR. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2004; 128:1224–9.

6. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002; 287:2114–9.

7. Bulten J, de Wilde PC, Boonstra H, Gemmink JH, Hanselaar AG. Proliferation in “atypical” atrophic Pap smears. Gynecol Oncol. 2000; 79:225–9.

8. Saminathan T, Lahoti C, Kannan V, Kline TS. Postmenopausal squamous-cell atypias: a diagnostic challenge. Diagn Cytopathol. 1994; 11:226–30.

9. Solomon D, Schiffman M. Tarone RALTS Study group. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance: baseline results from a randomized trial. J Natl Cancer Inst. 2001; 93:293–9.

10. ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol. 2003; 188:1383–92.
11. Siebers AG, Arbyn M, Melchers WJ, van Kemenade FJ, Vedder JE, van der Linden H, et al. Effectiveness of two strategies to follow-up ASC-US and LSIL screening results in the Netherlands using repeat cytology with or without additional hrHPV testing: a retrospective cohort study. Cancer Causes Control. 2014; 25:1141–9.

12. Wen CP, Tsai SP, Chung WS. A 10-year experience with universal health insurance in Taiwan: measuring changes in health and health disparity. Ann Intern Med. 2008; 148:258–67.

13. Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000; 29:336–43.

14. Cheng WF, Huang CY, You SL, Chen CJ, Hu CH, Chen CA. Clinical significance of cytologic atypical squamous cells of undetermined significance. Obstet Gynecol. 2009; 113:888–94.

15. Ostör AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993; 12:186–92.
16. Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O'Reilly S, et al. Early natural history of incident, type-specific human papillomavirus infections in newly sexually active young women. Cancer Epidemiol Biomarkers Prev. 2011; 20:699–707.

17. Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med. 1998; 338:423–8.

18. Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst. 1999; 91:252–8.

19. Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the effectiveness of cryotherapy in the treatment of cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2013; 120:218–23.

20. Chumworathayi B, Thinkhamrop J, Blumenthal PD, Thinkhamrop B, Pientong C, Ekalaksananan T. Cryotherapy for HPV clearance in women with biopsy-confirmed cervical low-grade squamous intraepithelial lesions. Int J Gynaecol Obstet. 2010; 108:119–22.

21. Poomtavorn Y, Suwannarurk K, Thaweekul Y, Maireang K. Diagnostic value of endocervical curettage for detecting dysplastic lesions in women with atypical squamous cells of undetermined significance (ASC-US) and low grade squamous intraepithelial lesion (LSIL) Papanicolaou smears. Asian Pac J Cancer Prev. 2014; 15:3461–4.

22. Jakobsson M, Gissler M, Paavonen J, Tapper AM. Loop electrosurgical excision procedure and the risk for preterm birth. Obstet Gynecol. 2009; 114:504–10.

23. Bevis KS, Biggio JR. Cervical conization and the risk of preterm delivery. Am J Obstet Gynecol. 2011; 205:19–27.

24. de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007; 7:453–9.

25. Chiang YC, Cheng WF, Chen YL, Chang MC, Hsieh CY, Lin MC, et al. High-risk human papillomavirus, other than type 16/18, in predominantly older Taiwanese women with high-grade cervical preinvasive lesions. Taiwan J Obstet Gynecol. 2013; 52:222–6.

26. Hammer A, Mejlgaard E, Gravitt P, Høgdall E, Christiansen P, Steiniche T, et al. HPV genotype distribution in older Danish women undergoing surgery due to cervical cancer. Acta Obstet Gynecol Scand. 2015; 94:1262–8.

27. Chen CA, Liu CY, Chou HH, Chou CY, Ho CM, Twu NF, et al. The distribution and differential risks of human papillomavirus genotypes in cervical preinvasive lesions: a Taiwan Cooperative Oncologic Group Study. Int J Gynecol Cancer. 2006; 16:1801–8.

28. Bentley JSociety of Canadian Colposcopists. Colposcopic management of abnormal cervical cytology and histology. J Obstet Gynaecol Can. 2012; 34:1188–202.
29. Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013; 17:S1–27.

30. Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papnicolaou results. JAMA. 1999; 281:1605–10.
Go to : 
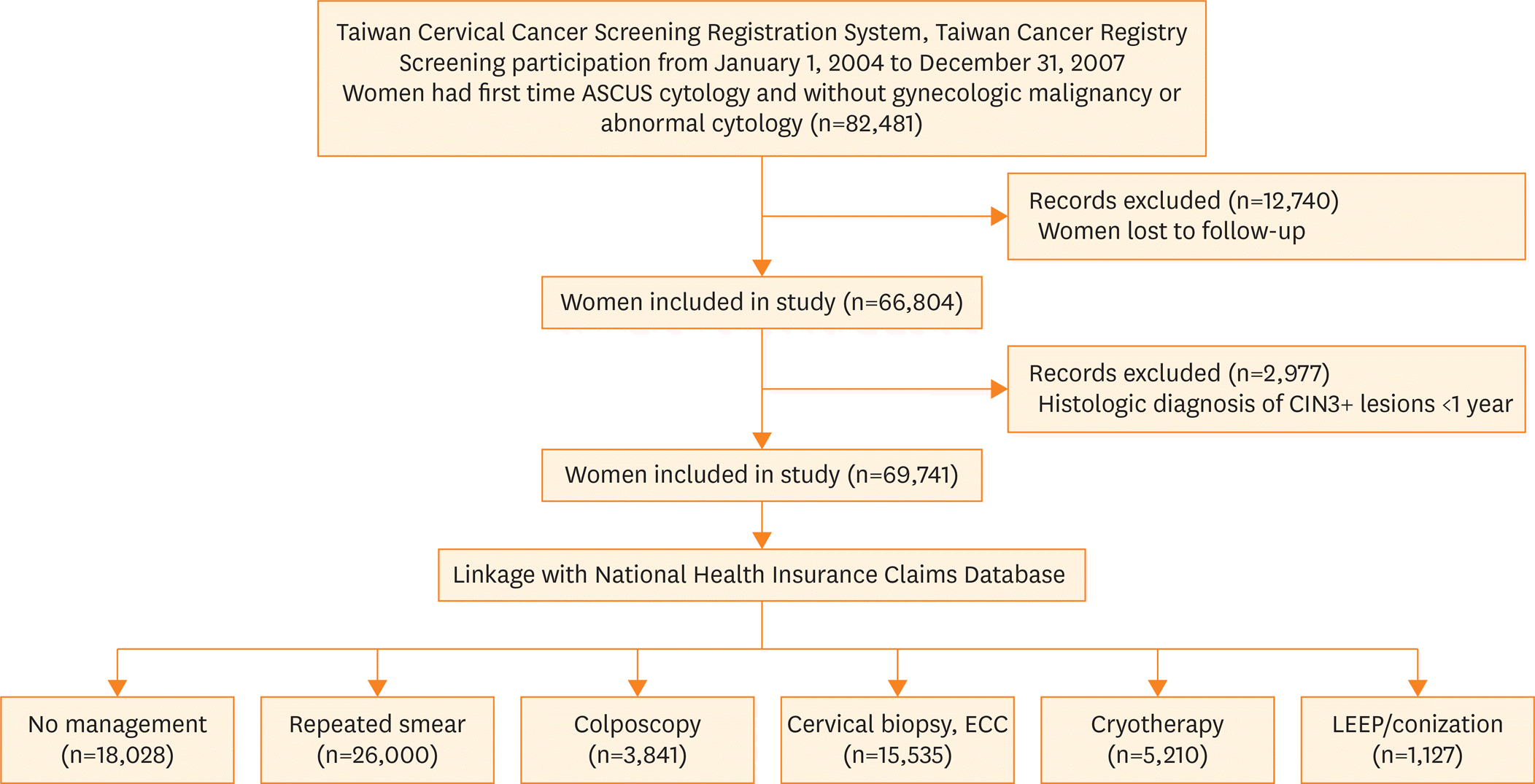 | Fig. 1.Flow of the study population among all women who attended cervical cancer screening with ASCUS cytology during 2004–2007. ASCUS, atypical squamous cells of undetermined significance; CIN3+, cervical intraepithelial neoplasia grade 3 and invasive cervical cancer; ECC, endocervical curettage; LEEP, loop electrosurgical excision procedure. |
 | Fig. 2.Survival analysis of subsequent risk for CIN3+ diagnosis in 69,741 women with ASCUS cytology according to management. X-axis refers to follow-up time in months since 1 year after the ASCUS cytology. Y-axis refers to cumulative incidence of subsequent CIN3+. ASCUS, atypical squamous cells of undetermined significance; CIN3+, cervical intraepithelial neoplasia grade 3 and invasive cervical cancer; ECC, endocervical curettage; LEEP, loop electrosurgical excision procedure. |
Table 1.
Baseline characteristics of 69,741 women with ASCUS cytology according to age distribution, screening interval, educational status, management, and incidence of subsequent CIN3+
| Characteristics | No. of ASCUS patients | Person-years of follow-up | No. of CIN3 | No. of invasive cancer | No. of p CIN3+ | Incidence of CIN3+ per 100,000 person-years* |
|---|---|---|---|---|---|---|
| Total | 69,741 | 266,011 | 669 | 103 | 772 | 290.2 |
| Age (yr) | ||||||
| 20–29 | 6,136 | 24,263 | 71 | 3 | 74 | 305.0 |
| 30–39 | 19,193 | 73,320 | 227 | 29 | 256 | 349.2 |
| 40–49 | 22,448 | 85,621 | 179 | 42 | 221 | 258.1 |
| 50–59 | 13,999 | 52,798 | 100 | 19 | 119 | 225.4 |
| 60–69 | 5,423 | 20,580 | 68 | 5 | 73 | 354.7 |
| >70 | 2,542 | 9,429 | 24 | 5 | 29 | 307.6 |
| Previous screening interval (yr) | ||||||
| <1 | 15,368 | 60,333 | 105 | 22 | 127 | 210.5 |
| 1–3 | 31,505 | 118,413 | 307 | 23 | 330 | 278.7 |
| 3–5 | 7,927 | 29,703 | 84 | 23 | 107 | 360.2 |
| >5 or never | 14,941 | 57,562 | 173 | 35 | 208 | 361.3 |
| Educational status | ||||||
| <6 years of schooling | 15,210 | 58,696 | 146 | 28 | 174 | 296.4 |
| Junior high school | 11,923 | 45,686 | 115 | 19 | 134 | 293.3 |
| Senior high school | 24,541 | 93,593 | 242 | 45 | 287 | 306.6 |
| College or graduate school | 17,412 | 65,567 | 165 | 11 | 176 | 268.4 |
| Management | ||||||
| No management | 18,028 | 68,557 | 198 | 59 | 257 | 374.9 |
| Non-interventional procedures | ||||||
| Repeated Pap smear | 26,000 | 99,659 | 232 | 27 | 259 | 259.9 |
| Colposcopy | 3,841 | 14,208 | 28 | 4 | 32 | 225.2 |
| Cervical biopsy and/or ECC | 15,535 | 58,765 | 172 | 6 | 178 | 302.9 |
| Interventional procedures | ||||||
| Cryotherapy | 5,210 | 20,267 | 36 | 7 | 43 | 212.2 |
| LEEP/conization | 1,127 | 4,555 | 3 | 0 | 3 | 65.9 |
Table 2.
Multivariate analysis of the risk of subsequent CIN3+ lesion in 69,741 women with ASCUS cytology




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download