Abstract
Backgrounds/Aims
Traditionally, surgically placed pancreatic drains are removed, at the discretion of the operating surgeon. Moving towards enhanced recovery after surgery (ERAS), we looked for predictors for early drain removal. The purpose of this paper was to establish which postoperative days' (POD) drain amylase is most predictive against post-operative pancreatic fistula (POPF).
Methods
We conducted a retrospective study of 196 patients who underwent pancreatic resection at our institute from January 2006 to October 2013. Drain amylase levels were routinely measured. The International Study Group of Pancreatic Fistula (ISGPF) definition of POPF, and clinical severity grading were used.
Results
5.1% (10 of 196) patients developed ISGPF Grades B and C POPF. Negative predictive value of developing significant POPF, if drain amylase values were low on PODs 1 and 3 was 98.7% (95% CI: 0.93–1.00). This translated to confidence in removing surgically placed pancreatic drains, at POD 1 and 3 when drain amylase values are low.
Pancreatoduodenectomy (PD) is a challenging and complex surgery. Morbidity after PD is as high as 65%.1 Pancreatic anastomosis is the Achilles heel of PD and determines surgical outcomes. Post-operative pancreatic fistula (POPF) occurs, in 5–30% of patients undergoing pancreatic resections. Incidence rates vary according to type of pancreatic resection, and definitions of POPF used.23 POPF is associated with significant morbidity such as intra- abdominal collections, intra- abdominal hemorrhage, and multi- organ failure. Associated mortality from POPF is 0–5%.4 Internationally, much work has been done, to understand risk factors for POPF in an effort to prevent, reduce, diagnose early, and manage POPF effectively. Contributory factors are multifactorial, including patient, disease, and procedure related factors. Patient-related factors include age, gender, jaundice, and malnutrition. Disease-related factors include pancreatic pathology, pancreatic texture, pancreas fat content, pancreatic duct size, and pancreatic juice output. Procedure- related factors include operative time, resection type, anastomotic technique, and intraoperative blood loss.1
Traditionally, surgeons leave behind drains in proximity to pancreatic anastomosis, to drain leaking contents and leave this for a variable period. The debate of ‘to drain’ or ‘not to drain’ following a major abdominal procedure, has endured for eternity and PD is no exception. Proponents of drains quote ‘it is safer to place a drain during an operation rather than after’. Opponents of drain quote ‘drain drains away the confidence of a surgeon!’ In a meta-analysis of one randomized trial and 7 observational studies including 2,690 patients, Zhou et al.5 reported that prophylactic intra-peritoneal drainage after pancreatic resection appears to be unable to improve the postoperative course, and may be associated with more severe and higher rate of complication and increased POPF occurrence. However, due to bias in included studies, further randomized or propensity score matched studies are needed, prior to any recommendation to change clinical practice. A recent global survey of experienced pancreatic surgeons reported that 14% never leave drains, 27% use drains selectively, and, of those who place drains, 51% reportedly removed drains early.6 In a recent systematic review including 14 randomized and non-randomized studies comparing routine intra-abdominal drainage versus no drainage, selective drain use, and early versus late drain removal after pancreatectomy, with major complications as primary outcome, Villafane-Ferriol et al.7 concluded that based on available evidence, the most conservative approach, pending further data, is routine placement of a drain and early removal, unless the patient's clinical course or drain fluid amylase concentration suggests a developing fistula.
Locally, it is our policy to insert two drains in proximity to the pancreatic anastomosis and these are kept for variable periods at the discretion of the operating surgeon; albeit all surgeons practice early removal of drains. With our unit moving towards enhanced recovery after surgery (ERAS), we evaluate predictors for early removal of drains, such that fit patients can be discharged earlier.89
The purpose of this project is to establish which post-operative day drain amylase is most accurate, in ruling out presence of pancreatic fistulas. The secondary end point is to examine other factors that increase odds of developing postoperative pancreatic fistula (POPF).
This is a retrospective, single-center study in an Asian population. From January 2006 to October 2013, 196 consecutive pancreatic resections were registered, in a specialized pancreatic unit database. Demographic profile, operative outcomes, pathologic reports, post-operative intervention, and outcome data were recorded. All patients who underwent pancreatic resection, were included in the study.
POPF was defined according to the updated International Study Group of Pancreatic Fistula (ISGPF) definition10 in 2016. POPF is defined as a drain output in a measurable volume of fluid, on or after postoperative day (POD) 3, with an amylase value greater than 3 times the upper limit of serum amylase value, associated with a clinically relevant development/condition related directly to POPF. The former 2005 classification of Grade A POPF is now redefined, and termed a ‘biochemical leak’. Definitions of POPF dependent on clinical impact are:2,11,12,13 1) Grade B POPF: Need for parenteral/enteral nutritional support. Patient may be kept nil by mouth. Percutaneous drainage may be required, and discharge may be delayed; 2) Grade C POPF: Medical and surgical intervention are required, and patient may require stabilization in the intensive care unit. Hospital stay is prolonged.
Drain amylase levels and values are routinely monitored for patients with pancreatic resection in our institution at post-operative days 1, 3, 5, and 7. Surgically placed pancreatic drains were kept on active suction via a drain bottle, and were kept until drain levels were less than 50 ml/day, and when drain amylase values were low (<3× serum amylase values).
All pancreatic resections were performed by 5 hepatopancreatobiliary surgery specialists
Octreotide is not routinely used in post-operative management of pancreatic resections in our institution. However, in patients wherein the pancreas was soft, the pancreatic duct was small, or where pancreato-enteric anastomosis was deemed to be ‘less ideal’ intra-operatively, octreotide was selectively used by surgeons.
Length of stay was defined as duration of an episode of hospitalization, from the date of pancreatic resection, to the date the patient was discharged from the hospital. Intervention was defined as any form of additional percutaneous or invasive procedure, that the patient had to undergo in addition to primary surgery, but not including surgical intervention. Re-operation was defined as an unplanned repeat operation related to primary surgery. Re-admission was defined as a repeat admission within 30 days of discharge from the primary admission, wherein pancreatic resection was performed. Mortality within 30 days of pancreatic resection, and mortality within the same inpatient stay for pancreatic resection, were studied.
Presence of a post-operative pancreatic fistula was correlated with patient demographics, standard clinicopathological parameters, and clinical outcome using the Chi-squared test, t test, and one-way ANOVA as appropriate. Univariate analyses were performed with Graphpad Prism version 7 (GraphPad software Inc., San Diego, CA, USA). Results were statistically significant if p was <0.05.
The most accurate post-operative point in detailing absence of POPF was determined by evaluating negative predictive values, when correlating a serum amylase value of more than 3 times normal (on POD 1, POD 1 and 3, POD 1, 3 and 5, and POD 3 and 5), with presence of a POPF. Highest negative predictive value with the least number of days of monitoring of drain amylase value, was the most accurate period.
Patients' demographic and clinical profiles are described in Table 1. There were a total of 126 pancreatoduodenectomies, 67 distal pancreatectomies, 2 pancreatic enucleations, and 1 central pancreatectomy (Table 2). Of 196 patients, 5.10% (10 of 196) patients developed a POPF (ISGPF Grades B & C). 42.3% (83 of 196 patients) had biochemical leak. 4.59% (9 of 196 patients) required percutaneous drainage (Grade B), while 0.51% (1 of 196 patients) had severe clinical sequelae from pancreatic fistula (Grade C). Drain amylase values were noted highest on POD 1. This trended down over the post-operative recovery period. Median number of days surgical drains were left in situ in those with significant POPF, compared with those with only biochemical leak and no POPF, was the same (8 days) (Table 2).
There were 93 patients with biochemical POPF, and 103 patients with no evidence of POPF. Ten of the patients with biochemical POPF, had significant POPF. Median length of stay, was 11 days for both groups (Table 2). Type of pancreatectomy, texture of pancreas, diameter of pancreatic duct, intra-operative blood loss, and use of octreotide, had no statistical impact on occurrence of POPF (Table 3).
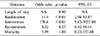
Patients with significant POPF had increased odds of being re- admitted (p<0.01). They were also found to have statistically higher need for procedural intervention, as compared to those without POPF (p<0.01). There was no significant difference in the need for re-operation (p=0.34) or inpatient death (p=0.36) (Table 4). Clinical outcome was examined in those with POPF Grades B and C (n=10), compared to those who had no POPF, or purely biochemical POPF (n=186) (Table 4). Median length of stay was 12 days, for those with significant POPF compared to 11 days in those without.
One of 10 patients with POPF Grades B and C underwent re-operation for exploratory laparotomy and drainage of abdominal collection. Five of 186 patients with no significant POPF underwent a re-operation for haemostasis, adhesiolysis, and secondary closure of the abdominal wall. This was not statistically significant.
There was also no statistical difference in occurrence of death in both groups. There were neither 30- day mortality nor inpatient mortality, from the group with POPF Grade B and C. This may be due to the small number (n=10). There was one 30-day mortality from the POPF Grade A group, and none from the no POPF group. The patient died from hypertensive heart disease. There were 3 other patients who had inpatient mortalities beyond 30 days, from the time of operation from the no POPF group. Their primary cause of death was pneumonia.
Patients with drain amylase values lower than 3 times that of serum amylase values at POD 1 and 3, were assessed with greatest negative predictive value, for developing POPF Grades B and C at 98.7% (95% CI 0.93 to 1.00) (Table 5).
Of the 196 patients who underwent pancreatic resections, 67 patients underwent distal pancreatectomy, and 126 patients underwent a PD.
8.95% (6 of 67) of patients who underwent a distal pancreatectomy, and 3.17% (4 of 126) patients who underwent a PD had significant POPF.
Patients who underwent distal pancreatectomy had 3 times the odds of having significant POPF, as compared to those who underwent a PD, but this was not statistically significant (p=0.10) (Table 3).
Use of octreotide and intraoperative blood loss, did not appear to affect incidence of POPF.
Further subgroup analysis of those who underwent PD, and distal pancreatectomies were performed.
Of 126 patients who underwent PD, there were 94 pancreato-jejunostomies (PJ), and 13 pancreato- gastrostomies (PG). Nineteen patients had no specified anastomosis. There was no statistical difference in occurrence of POPF from the type of pancreto-enteric anastomosis performed (p=1.0). Median diameter of the pancreatic duct in patients with POPF was 2.5 mm, while it was 3mm in those who did not have POPF. A smaller pancreatic duct did not show statistical significance (p=0.27) in predicting for POPF. Presence of soft pancreas intraoperatively (p=0.12) and use of octreotide (p=1.0) also did not affect occurrence of POPF (Table 6).
Risk factors for POPF were evaluated in those with distal pancreatectomy (n=67). A firm or thickened pancreas did not have statistical effect on occurrence of POPF (p=0.43). Use of octreotide (p=0.66), a method of closure of a stump with staples (p=0.68) or suture closure (p=1.0), also did not have statistical effect on occurrence of POPF (Table 6).
Using updated 2016 ISGPF criteria for POPF, incidence of POPF (Grades B and C) was 5.05% - Grade B POPF 4.55%, and Grade C 0.51%. This is lower than reported rates of 10–15.3%.214 When using the 2005 ISGPF definition, incidence of Grade A POPF was 42.3%.
The 2017 Cochrane review reported great variability in reporting of POPF, in terms of operative days and different cut-off values.15 Some studies identified a patient with pancreatic fistula after radiological confirmation, before using ISGPF classification to stratify POPF. Others used drain amylase value cut-offs >1000 U/L to >4000 U/L on POD 1, to define POPF.16 In our study, biochemistry was used as primary assessment for presence of POPF, and only patients with clinical signs and biochemically raised drain amylase value at POD 3 or after, had computed tomography to confirm presence of POPF.
We used the ISGPF definition of POPF in our study to predict for presence of POPF, having consistently studied drain amylase values on post-operative days 1, 3, 5, and 7. However, we discovered that this did not include the entire population of patients with POPF, as some patients were discovered with normal initial post-operative drain amylase values, but had peaked drain amylase values meeting ISGPF definition of POPF many days later. It was not practical to collect all potential POD drain amylase values, such as post-operative day 20 or day 35 drain amylase values, to capture the entire population with POPF. Two patients were noted with POPF when drained peri-pancreatic collections noted on computed tomography performed for clinical signs, showed significantly raised amylase values, but had initially low drain amylase values on POD 1 and 3. Use of ISGPF definition in our study to predict for presence of POPF, would guide us in how applicable ISGPF definition is in our population. It then enables us to determine, how it will guide our drain removal in the future.
There was statistically significant increased rate of re-admissions and interventions required in patients with POPF Grades B and C, as compared to those with no POPF, including those with biochemical leaks, in keeping with reported literature.101113
Molinari et al.14 described in a prospective study of 137 patients who underwent pancreatic resections, overall incidence of POPF (Grades A, B, and C) of 19.7%. When considering only POPF grades B and C, this was further broken down to 13.8% after distal pancreatectomy, and 9.9% after PD. This is in keeping with our study, wherein patients who underwent distal pancreatectomy were more likely to have POPF, as compared to after a PD (8.95% vs. 3.17%), although this was not statistically significant (p=0.10). Texture of pancreas and size of the pancreatic duct, did not have statistical significance on occurrence of POPF.
Negative predictive value for developing Grades B and C POPF at POD 1 alone was 100%. Negative predictive value of low drain amylase values at POD 1 and 3 was 98.7%, and was lower for those at POD 1, 3 and 5, and that of PODs 3, and 5. We took 98.7% as the most accurate day, as there were some patients with normal drain amylase values, but had collections high in amylase value when percutaneously drained, on POD 3 or after.
Previous studies1417 by Tsujie et al.17 have reported that patients with low drain amylase level on post-operative day 1 are safe from developing POPF.16 This translates to improved confidence in removing surgically-placed pancreatic drains at POD 3, in patients with low drain amylase values at POD 1 and 3. This is in contrast, to our current practice of keeping the surgical drain for an average of 7 days, in those without POPF. Consequently, this group of patients have potential of earlier discharge from the hospital, thus decreasing length of stay in the hospital. An earlier removal of surgically placed drains has also been reported to decrease rates of ascending infection, pancreatic fistula, and abdominal complications.718
Limitations of this study include the retrospective nature, varied practices among the 5 surgeons involved, single centre experience, span over 8 years during which various methods of resections and techniques as well as perioperative care have evolved. As a result, this study is also heterogeneous, including all types of pancreatic resections, and benign as well as malignant conditions. Further subgroup analysis was performed on the two main subgroups - PD, and distal pancreatectomy - to enhance understanding of these common procedures.
Presence of POPF has adverse outcome on a patient's post pancreatic resection. We recommend routine drain amylase level measurements post pancreatic resection. Negative predictive value of developing POPF Grade B and C, if drain amylase values were low on PODs 1 and 3, was 98.7%. Patients with low drain amylase values on POD 1 and 3, are unlikely to develop POPF. This translates to confidence in removal of surgically-pancreatic drains earlier on POD 3 if drain amylase levels are low, contributing to enhanced recovery after surgery.
Figures and Tables
Table 4
Univariate analysis of significant POPF causing worsened outcome in all patients who underwent pancreatic resections
References
1. Machado NO. Pancreatic fistula after pancreatectomy: definitions, risk factors, preventive measures, and management—review. Int J Surg Oncol. 2012; 2012:602478.
2. Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM Jr. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007; 245:443–451.

3. Schmidt CM, Powell ES, Yiannoutsos CT, Howard TJ, Wiebke EA, Wiesenauer CA, et al. Pancreaticoduodenectomy: a 20-year experience in 516 patients. Arch Surg. 2004; 139:718–725. discussion 725-727.
4. Schäfer M, Müllhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002; 236:137–148.

5. Zhou Y, Zhang X, Wu L, Ye F, Su X, Li B. Evidence-based value of prophylactic intraperitoneal drainage following pancreatic resection: a meta- analysis. Pancreatology. 2014; 14:302–307.
6. McMillan MT, Malleo G, Bassi C, Sprys MH, Vollmer CM Jr. Defining the practice of pancreatoduodenectomy around the world. HPB (Oxford). 2015; 17:1145–1154.

7. Villafane-Ferriol N, Shah RM, Mohammed S, Van Buren G 2nd, Barakat O, Massarweh NN, et al. Evidence-based management of drains following pancreatic resection: a systematic review. Pancreas. 2018; 47:12–17.
8. Robertson N, Gallacher PJ, Peel N, Garden OJ, Duxbury M, Lassen K, et al. Implementation of an enhanced recovery programme following pancreaticoduodenectomy. HPB (Oxford). 2012; 14:700–708.

9. Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced recovery after surgery (ERAS) protocols: time to change practice? Can Urol Assoc J. 2011; 5:342–348.

10. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an International Study Group (ISGPF) definition. Surgery. 2005; 138:8–13.

11. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017; 161:584–591.
12. Bassi C, Buchler MW, Fingerhut A, Sarr M. Predictive factors for postoperative pancreatic fistula. Ann Surg. 2015; 261:e99.

13. Hackert T, Hinz U, Pausch T, Fesenbeck I, Strobel O, Schneider L, et al. Postoperative pancreatic fistula: we need to redefine grades B and C. Surgery. 2016; 159:872–877.

14. Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, et al. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007; 246:281–287.
15. Davidson TB, Yaghoobi M, Davidson BR, Gurusamy KS. Amylase in drain fluid for the diagnosis of pancreatic leak in post-pancreatic resection. Cochrane Database Syst Rev. 2017; 4:CD012009.

16. Lu X, Wang X, Fang Y, Chen H, Peng C, Li H, et al. Systematic review and meta-analysis of pancreatic amylase value on postoperative day 1 after pancreatic resection to predict post-operative pancreatic fistula. Medicine (Baltimore). 2016; 95:e2569.

17. Tsujie M, Nakamori S, Miyamoto A, Yasui M, Ikenaga M, Hirao M, et al. Risk factors of pancreatic fistula after pancreaticoduodenectomy - patients with low drain amylase level on postoperative day 1 are safe from developing pancreatic fistula. Hepatogastroenterology. 2012; 59:2657–2660.

18. Kawai M, Tani M, Terasawa H, Ina S, Hirono S, Nishioka R, et al. Early removal of prophylactic drains reduces the risk of intra-abdominal infections in patients with pancreatic head resection: prospective study for 104 consecutive patients. Ann Surg. 2006; 244:1–7.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download