1. Goldstein I, Lue TF, Padma-Nathan H, Rosen RC, Steers WD, Wicker PA. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med. 1998; 338:1397–1404.
2. Park JH, Cho IC, Kim YS, Kim SK, Min SK, Kye SS. Body mass index, waist-to-hip ratio, and metabolic syndrome as predictors of middle-aged men's health. Korean J Urol. 2015; 56:386–392.
3. Canat L, Canat M, Guner B, Gurbuz C, Caşkurlu T. Association between renal function, erectile function and coronary artery disease: detection with coronary angiography. Korean J Urol. 2015; 56:76–81.
4. Ko K, Yang DY, Lee WK, Kim SW, Moon DG, Moon KH, Park NC, Park JK, Son HC, Lee SW, et al. Effect of improvement in lower urinary tract symptoms on sexual function in men: tamsulosin monotherapy vs. combination therapy of tamsulosin and solifenacin. Korean J Urol. 2014; 55:608–614.
5. DeLay KJ, Haney N, Hellstrom WJ. Modifying risk factors in the management of erectile dysfunction: a review. World J Mens Health. 2016; 34:89–100.
6. Ryu JK, Cho KS, Kim SJ, Oh KJ, Kam SC, Seo KK, Shin HS, Kim SW. Korean Society for Sexual Medicine and Andrology (KSSMA) guideline on erectile dysfunction. World J Mens Health. 2013; 31:83–102.
7. Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, Vardi Y, Wespes E; European Association of Urology. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010; 57:804–814.
8. Yuan J, Zhang R, Yang Z, Lee J, Liu Y, Tian J, Qin X, Ren Z, Ding H, Chen Q, et al. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013; 63:902–912.
9. Gresser U, Gleiter CH. Erectile dysfunction: comparison of efficacy and side effects of the PDE-5 inhibitors sildenafil, vardenafil and tadalafil--review of the literature. Eur J Med Res. 2002; 7:435–446.
10. Goldstein I, McCullough AR, Jones LA, Hellstrom WJ, Bowden CH, Didonato K, Trask B, Day WW. A randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of avanafil in subjects with erectile dysfunction. J Sex Med. 2012; 9:1122–1133.
11. Jung J, Choi S, Cho SH, Ghim JL, Hwang A, Kim U, Kim BS, Koguchi A, Miyoshi S, Okabe H, et al. Tolerability and pharmacokinetics of avanafil, a phosphodiesterase type 5 inhibitor: a single- and multiple-dose, double-blind, randomized, placebo-controlled, dose-escalation study in healthy Korean male volunteers. Clin Ther. 2010; 32:1178–1187.
12. Mulhall JP, Burnett AL, Wang R, McVary KT, Moul JW, Bowden CH, DiDonato K, Shih W, Day WW. A phase 3, placebo controlled study of the safety and efficacy of avanafil for the treatment of erectile dysfunction after nerve sparing radical prostatectomy. J Urol. 2013; 189:2229–2236.
13. Kedia GT, Uckert S, Assadi-Pour F, Kuczyk MA, Albrecht K. Avanafil for the treatment of erectile dysfunction: initial data and clinical key properties. Ther Adv Urol. 2013; 5:35–41.
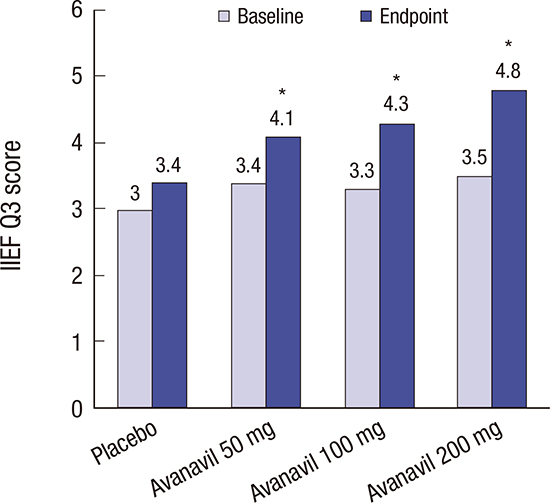
14. Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002; 14:226–244.
15. Hellstrom WJ, Freier MT, Serefoglu EC, Lewis RW, DiDonato K, Peterson CA. A phase II, single-blind, randomized, crossover evaluation of the safety and efficacy of avanafil using visual sexual stimulation in patients with mild to moderate erectile dysfunction. BJU Int. 2013; 111:137–147.
16. Carvalheira AA, Pereira NM, Maroco J, Forjaz V. Dropout in the treatment of erectile dysfunction with PDE5: a study on predictors and a qualitative analysis of reasons for discontinuation. J Sex Med. 2012; 9:2361–2369.
17. Ljunggren C, Hedelin H, Salomonsson K, Ströberg P. Giving patients with erectile dysfunction the opportunity to try all three available phosphodiesterase type 5 inhibitors contributes to better long-term treatment compliance. J Sex Med. 2008; 5:469–475.
18. Leungwattanakij S, Flynn V Jr, Hellstrom WJ. Intracavernosal injection and intraurethral therapy for erectile dysfunction. Urol Clin North Am. 2001; 28:343–354.
19. Vardi Y, Sprecher E, Gruenwald I. Logistic regression and survival analysis of 450 impotent patients treated with injection therapy: long-term dropout parameters. J Urol. 2000; 163:467–470.
20. Sundaram CP, Thomas W, Pryor LE, Sidi AA, Billups K, Pryor JL. Long-term follow-up of patients receiving injection therapy for erectile dysfunction. Urology. 1997; 49:932–935.
21. Park NC, Kim TN, Park HJ. Treatment strategy for non-responders to PDE5 inhibitors. World J Mens Health. 2013; 31:31–35.
22. Wang H, Yuan J, Hu X, Tao K, Liu J, Hu D. The effectiveness and safety of avanafil for erectile dysfunction: a systematic review and meta-analysis. Curr Med Res Opin. 2014; 30:1565–1571.







 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download