Abstract
Continuous erythropoietin receptor activator (CERA) is an erythropoietin with a long-half life. This study investigated the efficacy of CERA for correcting anemia in Korean patients on dialysis. Patients (≥18 yr) who were not receiving any ESAs for more than 8 weeks were randomly assigned to either intravenous CERA once every 2 weeks (n=39) or epoetin beta thrice-weekly (n=41) during a 24-week correction phase. Hemoglobin (Hb) response was defined as increase of Hb by at least 1 g/dL and Hb≥11 g/dL without red blood cell (RBC) transfusion. Median dialysis duration was 1.7 (0.3-20.8) and 1.6 (0.4-13.8) yr in CERA and epoetin beta group, respectively. Hemoglobin response rate of CERA was 79.5% (95% confidence interval [CI], 63.5-90.7). As the lower limit of 95% CI was higher than pre-specified 60% response rate, it can be concluded that CERA corrected anemia (P<0.05). Hb response rate of epoetin beta was 87.8% (95% CI, 73.8-95.9) (P=0.37). Median time to response was 12 weeks in CERA and 10.3 weeks in epoetin beta (P=0.03). It is suggested that once every 2 weeks administration of CERA is effective for correcting anemia in Korean patients on long-term hemodialysis with longer time-to-response than thrice weekly epoetin beta.
Most patients with chronic kidney disease (CKD) develop anemia along with progressive loss of renal function. Anemia is caused by a relative deficiency of erythropoietin (EPO). After recombinant human EPO was introduced in 1989, exogenous replacement of EPO became the standard treatment of anemia in patients with CKD (1). However, difficulties in management of anemia have been involved with frequent administrations and dose changes of EPOs with short half-life. In patients with CKD on dialysis, treatment of anemia requires life-long replacement with EPOs. Therefore, EPO with extended half-life and sustained efficacy may improve the convenience and therapeutic utility of the drug.
Continuous erythropoietin receptor activator (CERA) is composed of epoetin beta chemically bound to a linear methoxy-polyethylene glycol moiety (2). In contrast with short-acting EPOs, CERA shows an increased half life, about 130 hr (3). The ability to use CERA and conventional EPOs, with correction or maintenance purposes has been examined in six phase III clinical trials (4-9). Among them, there was one phase III correction study which showed comparable efficacy with once every 2 weeks intravenous (IV) administration of CERA to that of 3 times weekly epoetin for hemoglobin (Hb) correction in EPO-naïve patients on dialysis (4). As this trial contained the patients on the relatively short-term dialysis and small number of Asian patients (less than 10%), there is a lack of evidence of efficacy and safety in general dialysis patients and also in Korean patient population.
This study was conducted to demonstrate the efficacy of CERA treatment for correction of anemia in Korean patients receiving dialysis.
This was a multi-center, phase III, open-label, randomized, prospective study with parallel group during 24-week correction phase; patients with once every 2 weeks IV CERA versus patients with three times per week IV epoetin beta. All enrolled patients were randomly assigned by 1:1 ratio to either CERA group or epoetin beta group. After correction phase, the responders from both groups were entered to 24-week maintenance phase of once monthly CERA IV treatment (Fig. 1).
The primary efficacy parameter was Hb response rate in the intention-to-treat (ITT) population. Hb response was defined as increase of Hb by at least 1 g/dL compared with baseline and Hb≥11 g/dL without red blood cell (RBC) transfusion during 24-week correction phase. Secondary efficacy parameters were; 1) mean hemoglobin levels and their changes over time from baseline, 2) time to Hb response, and 3) RBC transfusion rate during 24-week correction phase.
Safety parameters were vital signs, electrocardiograms (EKG), adverse events (AEs), safety laboratory parameters including iron, and anti-EPO antibody.
We screened patients (age≥18 yr) receiving regular dialysis at least for 4 weeks before randomization at 7 centers in Korea. To be included to the study, patients should be on adequate dialysis, that is, measured Kt/V ≥1.2 or urea reduction ratio (URR) ≥65% for hemodialysis (HD). We defined baseline predialysis hemoglobin as mean of all values recorded between the day of first study dose and the previous 20 days. It must be between 8 and 11 g/dL. Patients were also required to have adequate iron status, defined as serum ferritin ≥100 ng/mL or transferrin saturation (TSAT) ≥20% (or hypochromic red blood cells <10%). Patients were excluded if they had: previous therapy with recombinant human EPO or any other erythropoietic substance within 8 weeks prior to screening, overt bleeding which necessitated blood transfusion 8 weeks before screening or during screening, failure of kidney transplantation, uncontrolled and chronic inflammatory disease, chronic congestive heart failure (NYHA class IV), poorly controlled hypertension, uncontrolled secondary hyperparathyroidism, or life expectancy of less than 12 months.
After a 2-week screening period, eligible patients were randomized to receive either CERA or epoetin beta. The starting dose was 0.6 µg/kg of CERA IV once every 2 weeks. The starting dose of epoetin beta was 40 IU/kg IV, three times per week. During 24-week correction phase, dose adjustments were performed to achieve Hb level≥11 g/dL and an increase of ≥1 g/dL from each patient's baseline Hb. CERA doses were adjusted according to the predefined protocol, but no more often than once every 4 weeks. The predefined protocol as follows; 1) if the Hb increased by <1 g/dL during 4 weeks compared with baseline, doses were increased by 25%; 2) if Hb increased by >2 g/dL versus baseline or was between 12 g/dL and 13 g/dL, doses were decreased by 25%; 3) if Hb was greater than 13 g/dL, CERA should be stopped temporarily until Hb level decreased to less than 12 g/dL, then therapy was resumed at a dose 25% below the previously administered dose; 4) if Hb decreased to less than baseline value and was less than 9 g/dL, doses were increased by 50%. Dose adjustments of epoetin beta were performed according to each investigator's discretion, but not more often than once every 4 weeks.
At week 25, the responders in both study groups (defined as patients who have reached the target Hb at least once during the correction phase without RBC transfusion and completed correction phase treatment) entered to CERA IV once every 4 weeks treatment for 24 weeks (weeks 25-52). The starting dose of maintenance phase for patients who had received CERA during the correction phase was the double dose of week 23. The starting dose for patients who had received epoetin beta was determined by patients' previous weekly dose of epoetin beta. Starting doses of CERA were either 120, 200, or 360 µg for patients who had received either less than 8,000, 8,000-16,000, or more than 16,000 IU of epoetin beta per week, respectively. During 24-week maintenance phase, dose adjustments were performed to maintain Hb levels in a target range of 11-12 g/dL. IV iron supplementation was prescribed to prevent iron deficiency during treatment period according to each center's practice.
Patients were evaluated weekly during the screening, every 2 weeks during the correction phase, and every 4 weeks during maintenance phase and at the final visit. Hemoglobin, hematocrit, blood pressure and heart rate were measured at every visit. Iron and other safety laboratory parameters were measured during screening, and every 6 weeks during the correction phase, at weeks 25, 37 and at the final visit. Blood samples for detecting anti-EPO antibody were collected at randomization, weeks 13, 25 and at final visit and were sent to the central laboratory. Quality of life was inquired by using the Korean version of Short-Form-36 Health Survey at randomization and weeks 13 and 25.
For all patients who had received at least one dose of study drug, adverse events (AEs) were recorded. RBC transfusions, iron supplementation, and concomitant medications were recorded throughout the study.
This study was designed to test the hypothesis that the efficacy of IV CERA every 2 weeks for correction of anemia would be greater than 60% in dialysis patients. The epoetin beta group was included to provide reassurance that the results observed in CERA group are comparable to those seen under an approved EPO. The hypothesis was tested by calculating two-sided 95% confidence interval (CI) by Clopper-Pearson method. If the lower limit of two-sided 95% CI was greater than 60%, it could be concluded that every 2 weeks IV CERA treatment was effective for correction of anemia. The lower limit for the CI for the response rates was chosen to be 60%, based on information available at the time of the protocol development of the previous studies (4, 5). Analysis of the primary efficacy parameter was performed on the ITT population and was confirmed on the per-protocol (PP) population. Among the patients who needed RBC transfusion, the patient who achieved target Hb before RBC transfusion was included as responder. The sample size of 78 patients, 39 patients for each group, was required to prove the hypothesis with 80% power.
Secondary efficacy analyses were performed on the ITT population. Mean hemoglobin levels and their changes over time from baseline were presented. The time to Hb response was analyzed by using Kaplan-Meier method. The occurrence of RBC transfusions during 24 weeks was summarized using descriptive method in each group. The post-hoc statistical analyses were provided for primary and secondary efficacy outcomes using Fisher's exact test for hemoglobin response rate and RBC transfusions, repeated measures ANOVA for mean Hb levels and their changes over time and log-rank test for the time to Hb response.
Safety analyses were performed on all patients who received at least one dose of study medication irrespective of drop-out from the study. While analyses for the safety were done after all patients finished the final visit, primary and secondary efficacy parameters were analyzed only on the basis of the data from the correction phase. Results of continuous variables are presented as mean±SD.
This study protocol was reviewed and approved by institutional review board of Kangdong Sacred Heart Hospital (IRB No. 07-30) and the institutional review board of 6 other involved hospitals. All patients gave written informed consent before study entry. This trial was registered at ClinicalTrials.gov (www.clinicaltrial.gov), No. NCT00546481.
A total of 80 patients were randomized, 39 patients for CERA and 41 patients for epoetin beta, and were included in ITT analysis (Fig. 2). Three patients (1 assigned to CERA and 2 to epoetin beta) did not receive any study medication and were excluded from the safety analyses. Eleven patients withdrew during the correction phase, 6 from CERA and 5 from epoetin beta. Sixty-nine patients completed the correction phase (33 given CERA and 36 given epoetin beta). We excluded 22 patients in the PP analysis (13 given CERA and 9 given epoetin beta) who did not receive study drug or had major protocol violations such as insufficient Hb measurements (<75%), bleeding episodes, RBC transfusions or wrong/missed doses. Fifty eight patients were included for the PP analysis, 26 of CERA and 32 of epoetin beta group. Total 54 patients were eligible for maintenance phase; 25 patients from CERA group and 29 patients from epoetin beta group and 50 patients completed the study.
Baseline characteristics were similar between two study groups (Table 1). Body weight and body mass index (BMI) were higher in epoetin beta group. All of the patients were on HD and most of the patients had been on long-term dialysis. Median dialysis duration was 1.7 (0.3-20.8) yr and 1.6 (0.4-13.8) yr in CERA group and epoetin beta group, respectively. More patients in CERA group had arteriovenous graft; 7 (18.0%) vs 2 (4.9%) patients. Before the trial, 100% of the patients in both groups had previously received EPO treatment. Mean baseline Hb concentrations did not differ between groups, 9.00 vs 8.97 g/dL, respectively.
Hb response rates were 79.5% (95% CI, 63.5-90.7) and 87.8% (95% CI, 73.8-95.9) for CERA and epoetin beta, respectively (P=0.37) (Table 2). It demonstrated that CERA IV once every 2 weeks corrected anemia because the lower limit of the 95% CI for the CERA response rate was greater than the pre-defined 60% response.
For the secondary efficacy analysis, Fig. 3A shows biweekly mean Hb concentrations and their changes over time during the correction phase (P=0.18). During the first two weeks, mean Hb levels of both groups declined, and then they increased steadily towards the peak Hbs. At the end of correction phase, mean Hb increased by 2.0 g/dL from baseline in both groups. In epoetin beta group, more patients experienced overcorrection of Hb (greater than 13.0 g/dL), 9 (23%) in CERA and 15 (37%) in epoetin beta group.
Median time to response was 12 weeks (84.0 days; 95% CI, 70.0-98.0) in CERA and 10.3 weeks (72 days; 95% CI, 58.0-72.0) in epoetin beta (P=0.03) (Table 2). It showed that the correction of anemia was slower in CERA group compared with epoetin beta group. We got the similar results in the analysis of these parameters on the PP population set (data not shown).
More patients needed to receive at least one RBC transfusion in CERA group (8 patients, 20.5%, total 16 RBC transfusions) compared with in epoetin beta group (2 patients, 4.9%, total 10 RBC transfusions) during 24-week correction phase (P=0.05).
At week 13, a meaningful decrease (≥5 points) was observed on physical functioning for CERA group. At week 25, mean scores increased on all subscales except role-emotional for CERA group and clinically meaningful improvement was observed in vitality for CERA group (Fig. 4).
Overall and frequently reported AEs during the correction phase were comparable between the two study arms (Table 3). Arteriovenous fistula or graft complication was reported in 5 patients from each group during the correction phase. Two patients were reported with anemia and one patient withdrew due to insufficient therapeutic response from CERA group. One case from each group was classified as possibly related adverse events (headache in CERA; hypertension in epoetin beta). Serious adverse events were reported 14 events in 10 patients (26.3%) in CERA and 7 events in 6 patients (15.4%) in epoetin beta group. None of them was judged to be related with study drugs. During maintenance phase, 51 patients (94.4%) had at least one adverse event and 8 patients (14.8%) had serious adverse events. There was one patient with decreased Hb level and administered RBC transfusion. There were no deaths in both correction and maintenance phase. Anti-EPO antibody was not detected throughout the study.
This study demonstrated that intravenous CERA once every 2 weeks corrected renal anemia of long-term dialysis patients during 24 week correction phase. It was accompanied by longer time to response and higher RBC transfusion rate in CERA group than in epoetin beta group.
This study had similar study design to AMICUS study (4). Both studies were phase III, randomized, open-label, multicenter studies, and had a parallel group of IV epoetin alfa or beta three times a week. They were performed to evaluate the efficacy of correction of renal anemia with CERA IV every 2 weeks in patients on dialysis. In AMICUS study the starting dose was 0.4 µg/kg/2 weeks, and subsequent doses were decided in broader adjustment range (-50%). However, the results were different. Hb response rates were 79.5% (95% CI, 63.5-90.7) and 87.8% (95% CI, 73.8-95.9) for CERA and epoetin beta, respectively in the current study. This response rate of CERA group was lower than 93% (95% CI, 87.7-96.9) of CERA group in AMICUS study. Median time to response in both groups was relatively delayed in our patient population. It was 84 days in CERA group and 72 days in epoetin beta group, which was delayed by approximately 4 to 6 weeks when compared with those in AMICUS study; 57 days (95% CI, 50-64) with CERA and 31 days (95% CI, 27-38) with epoetin.
One of reasons for these discordances might be the difference of study population. Although both studies were conducted in patients with CKD on dialysis, median dialysis duration of patients in this study was 621 days and 590 days in CERA and epoetin beta group, respectively, which was much longer than those in AMICUS study; 166 days and 113 days in CERA and epoetin group respectively. All patients in this study had received EPO therapy before. In contrast, about 30% of patients had history of previous EPO treatment in AMICUS. Baseline hemoglobin concentrations were higher in patients of AMICUS study (9.39±0.88 g/dL and 9.40±0.82 g/dL in CERA and epoetin group, respectively) in spite of not receiving EPO in the previous 12 weeks. These facts suggest that overall residual renal function (RRF) in the patients of current study was much lower than that in AMICUS. RRF is known to decline following the initiation of HD. In retrospective study, Vilar et al. (10) reported the fraction of HD patients who had a residual renal urea clearance ≥ 1 mL/min. At 3 months after initiating HD, this proportion was 85.4%, but it decreased over time to 58.1% at 2 yr and 31.1% at 5 yr. In patients with RRF<1 mL/min, weekly EPO dose and EPO resistance index were significantly higher from 12 months after the start of HD than in patients with preserved RRF (10). The presence of RRF in chronic dialysis patients contributes to lower EPO requirement in HD patients. In a recent study, strong relation between RRF and improved anemia control in HD patients was reported, suggesting less EPO resistance in patients with high GFR (11). It could suggest that long-term dialysis patients might need a little higher starting dose of CERA than 0.6 µg/kg/2 weeks to overcome their high EPO resistance and this plausible reason might cause some part of the lower response rate and slower time to response in our study than the previous phase II or III correction studies. The starting dose of 0.6 µg/kg/2 weeks IV of CERA in this study was based on two phase III studies conducted in CKD patients on dialysis and not on dialysis, AMICUS and ARCTOS, respectively (4, 5). Based on this observation, dose of IV CERA started from 0.6 µg/kg/2 weeks in our study and followed the dosing protocol. Median dose at the time of Hb response went up to 0.9 µg/kg/2 weeks, which declined to 0.7 µg/kg/2 weeks at the end of correction phase (Table 4). After the first month there was a sharp increase in CERA dose by 33% in response to minimal increase of Hb level. The clinical studies of CERA have shown a dose-dependent erythropoietic response. In two phase II studies, serum concentrations of CERA were positively correlated with Hb response (12, 13). These studies also showed a trend towards more rapid Hb response was evident with higher dose.
Another potential reason for less Hb response rate and delayed time to response was iron supplementation. Baseline median TSAT levels were about 40% in both groups and ferritin levels were 365 and 405 ng/mL in CERA and epoetin beta, respectively (Fig. 5). After the patients were administered study drugs, median TSAT declined to 26 and 28% and median ferritin dropped to 187 and 200 ng/mL at week 13 in CERA and epoetin beta, respectively. These parameters increased to a certain degree at the end of the correction phase. During the correction phase most patients have taken oral iron and IV iron treatments were intermittently prescribed in response to iron deficiency according to the criteria covered by national health insurance. Iron is essential to support erythropoiesis. Although EPO treatment is greatly effective in correcting Hb levels, absolute or functional iron deficiency causes a state of hyporesponsiveness to EPOs. Baseline iron parameters in our study were definitely in target range as recommended by current guidelines (14, 15), but iron levels decreased due to rapid consumption with initiation of EPO treatment. While most of the patients maintained TSAT in range of 20%-50% or serum ferritin in the range 100-800 ng/mL during the correction phase, there was some proportion of patients in iron deficiency, especially during the first 13 weeks. It might partially contribute to high dose of study drugs at the time of Hb response. These two reasons seem to be related with high occurrence of RBC transfusion in CERA group. Five patients in CERA group received RBC transfusion in early correction phase between week 3 and 9.
Fifty-four patients were included in maintenance phase of CERA every 4 weeks. During maintenance phase mean Hb concentrations were maintained between 11 to 12 g/dL (Fig. 3B), which means that high degree of Hb stability was achieved after direct conversion from CERA every 2 weeks or epoetin three time per week to CERA once-monthly schedule. While RBC transfusion rate was high in CERA group during the correction phase, only one patient (2%) was reported to receive RBC transfusion in the maintenance phase. Recent studies showed that conversion of a large population of HD patients from epoetin or darbepoetin to monthly CERA administration was shown to offer good control of Hb levels using pre-filled syringes (16) and once-monthly CERA therapy maintained stable Hb values with low intra-individual variability in HD patients under routine conditions (17).
We acknowledge the limitation of open-label study design, which may affect some parameters, such as quality of life or report of any AEs. As it was a small study, it had some limitation to reflect the results of the previous bigger ones.
In summary, CERA IV once every 2 weeks corrected renal anemia of prior-EPO treated, long-term hemodialysis patients during 24 week correction phase. CERA treatment was well tolerated throughout the study. Conversion to once-monthly IV CERA maintained mean hemoglobin levels between 11 and 12 g/dL during 24 week maintenance phase.
Figures and Tables
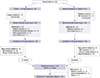 | Fig. 2Enrollment, randomization, and study completion. *RBC transfusion; §One patient from each group had sufficient Hb measurements (> 75%) during correction phase and was included in per protocol analysis. |
 | Fig. 3Mean hemoglobin levels. (A) Changes over time from baseline (BL) during the correction phase in CERA IV once every 2 weeks and epoetin beta IV three times per week group (ITT set) (P = 0.18, repeated measures ANOVA). (B) Changes during the maintenance phase. Values are mean ± SD. CERA, Continuous erythropoietin receptor activator. |
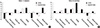 | Fig. 4Changes in quality of life evaluated by using SF-36 Health Survey. A clinically meaningful change is defined as a change greater than 5 points from baseline. (A) At week 13. (B) At week 25. CERA, Continuous erythropoietin receptor activator. |
 | Fig. 5Change of iron parameters during the correction phase. Values are median with interquartile range. (A) Transferrin saturation (TSAT) (P = 0.38, repeated measures ANOVA). (B) Ferritin (P = 0.94, repeated measures ANOVA). CERA, Continuous erythropoietin receptor activator. |
Table 1
Demographic and laboratory data at baseline (Intention-to-treat set)*

*Values are mean±SD or median with range. EPO, erythropoietin; BMI, body mass index; Kt/V, single pool Kt/V; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin II receptor blockers; TSAT, transferrin saturation; PTH, parathyroid hormone; CERA, Continuous erythropoietin receptor activator.
Table 2
Hemoglobin response rate and median time to response in CERA and epoetin beta group during the correction phase (ITT set)

ACKNOWLEDGMENTS
We appreciate all medical and nursing staff of dialysis units from Kangdong Sacred Heart Hospital, Hallym University Sacred Heart Hospital, Gachon Gil Hospital, Seoul National University Bundang Hospital, Samsung Medical Center, Seoul St. Mary's Hospital, and Seoul National University Hospital.
References
1. Henry DH, Bowers P, Romano MT, Provenzano R. Epoetin alfa: clinical evolution of a pleiotropic cytokine. Arch Intern Med. 2004; 164:262–276.
2. Curran MP, McCormack PL. Methoxy polyethylene glycol-epoetin beta: a review of its use in the management of anaemia associated with chronic kidney disease. Drugs. 2008; 68:1139–1156.
3. Macdougall IC, Robson R, Opatrna S, Liogier X, Pannier A, Jordan P, Dougherty FC, Reigner B. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006; 1:1211–1215.
4. Klinger M, Arias M, Vargemezis V, Besarab A, Sulowicz W, Gerntholtz T, Ciechanowski K, Dougherty FC, Beyer U. Efficacy of intravenous methoxy polyethylene glycol-epoetin beta administered every 2 weeks compared with epoetin administered 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomized trial. Am J Kidney Dis. 2007; 50:989–1000.
5. Macdougall IC, Walker R, Provenzano R, de Alvaro F, Locay HR, Nader PC, Locatelli F, Dougherty FC, Beyer U. ARCTOS Study Investigators. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin J Am Soc Nephrol. 2008; 3:337–347.
6. Levin NW, Fishbane S, Cañedo FV, Zeig S, Nassar GM, Moran JE, Villa G, Beyer U, Oguey D. MAXIMA Study Investigators. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA). Lancet. 2007; 370:1415–1421.
7. Sulowicz W, Locatelli F, Ryckelynck JP, Balla J, Csiky B, Harris K, Ehrhard P, Beyer U. PROTOS Study Investigators. Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007; 2:637–646.
8. Canaud B, Mingardi G, Braun J, Aljama P, Kerr PG, Locatelli F, Villa G, Van Vlem B, McMahon AW, Kerloëguen C, et al. Intravenous C.E.R.A. maintains stable haemoglobin levels in patients on dialysis previously treated with darbepoetin alfa: results from STRIATA, a randomized phase III study. Nephrol Dial Transplant. 2008; 23:3654–3661.
9. Spinowitz B, Coyne DW, Lok CE, Fraticelli M, Azer M, Dalal S, Villa G, Rosansky S, Adamis H, Beyer U. C.E.R.A. maintains stable control of hemoglobin in patients with chronic kidney disease on dialysis when administered once every two weeks. Am J Nephrol. 2008; 28:280–289.
10. Vilar E, Wellsted D, Chandna SM, Greenwood RN, Farrington K. Residual renal function improves outcome in incremental haemodialysis despite reduced dialysis dose. Nephrol Dial Transplant. 2009; 24:2502–2510.
11. Penne EL, van der Weerd NC, Grooteman MP, Mazairac AH, van den Dorpel MA, Nubé MJ, Bots ML, Lévesque R, ter Wee PM, Blankestijn PJ. Role of residual renal function in phosphate control and anemia management in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2011; 6:281–289.
12. Locatelli F, Reigner B. C.E.R.A.: pharmacodynamics, pharmacokinetics and efficacy in patients with chronic kidney disease. Expert Opin Investig Drugs. 2007; 16:1649–1661.
13. Fishbane S, Pannier A, Liogier X, Jordan P, Dougherty FC, Reigner B. Pharmacokinetic and pharmacodynamic properties of methoxy polyethylene glycol-epoetin beta are unaffected by the site of subcutaneous administration. J Clin Pharmacol. 2007; 47:1390–1397.
14. Locatelli F, Aljama P, Bárány P, Canaud B, Carrera F, Eckardt KU, Hörl WH, Macdougal IC, Macleod A, Wiecek A, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004; 19:ii1–ii47.
15. KDOQI. National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis. 2006; 47:S11–S145.
16. Fliser D, Kleophas W, Dellanna F, Winkler RE, Backs W, Kraatz U, Fassbinder W, Wizemann V, Strack G. Evaluation of maintenance of stable haemoglobin levels in haemodialysis patients converting from epoetin or darbepoetin to monthly intravenous C.E.R.A.: the MIRACEL study. Curr Med Res Opin. 2010; 26:1083–1089.
17. Weinreich T, Leistikow F, Hartmann HG, Vollgraf G, Dellanna F. SESAM Study Group. Monthly continuous erythropoietin receptor activator treatment maintains stable hemoglobin levels in routine clinical management of hemodialysis patients. Hemodial Int. 2012; 16:11–19.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download