Abstract
Backgrounds/Aims
After centralization policy, clinical outcomes have been improved in patients underwent pancreaticoduodenectomy for pancreatic cancer. However, centralization could exacerbate the prolongation of surgical waiting time. This study aims to investigate whether the shorter waiting time correlates with the better survival and to identify the major confounders that influence the association between those.
Methods
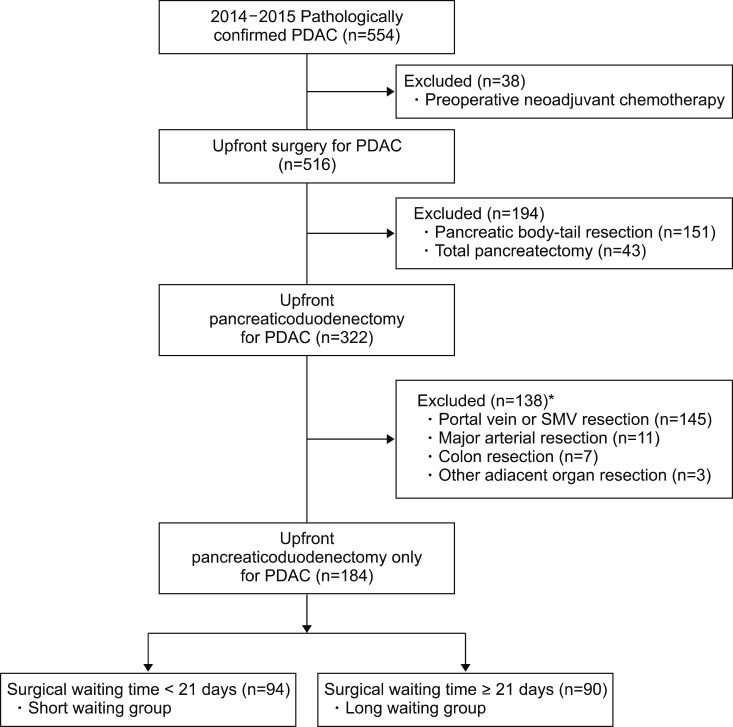
In this retrospective cohort study, a total 554 patients with pathologically confirmed pancreatic ductal adenocarcinoma were assessed the eligibility from 2014 through 2015. Patients with neoadjuvant chemotherapy, body-tail resection, total pancreatectomy and combined adjacent organ resection were excluded. All patients were divided into two groups by median waiting time, 21 days, defined as the date difference between initial imaging diagnosis and operation.
Results
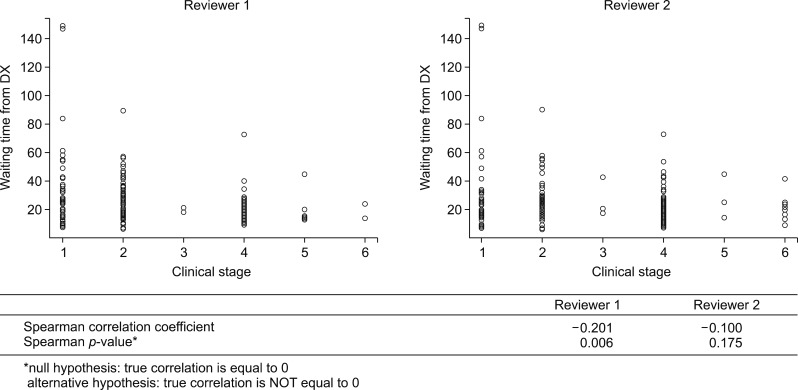
Median overall survival did not differ between long and short waiting group (30.4 vs 24.8 months, p=0.35; HR=0.84, 95% CI=0.58–1.21). The proportion of cancer stage shifting, the difference between clinical and pathologic staging, did not differ depending on waiting time group (p=0.811 and 0.255, each of reviewers). Short waiting time was highly correlated with high initial clinical stage (Spearman correlation coefficients −0.201 (p=0.006) and −0.100 (p=0.175), each of reviewers).
Conclusions
Initial clinical stage had confounding effect on the association between waiting time and overall survival. Therefore, in evaluating centralization policy at the national level, evidence for maximum acceptable waiting time should be investigated in the near future with considering that surgical waiting time could be affected by initial clinical stage.
Go to : 
With recent development in management, pancreatic cancer has shown significant improvement in prognosis.1 Surgery still represents the only potentially curative treatment, and many innovations have been suggested to improve the clinical outcome of pancreatic cancer.2 Hospital volume, in other words, centralization, is one of those innovations, and several studies have reported an association between hospital volume and clinical outcome.34
However, centralization could exacerbate a potential weakness: the prolongation of surgical waiting time. Delayed surgical resection for tumors can adversely affect tumor stage and survival in other types of cancer surgery, including lung, breast and colorectal cancers.567 Several reports have investigated the association between pancreatic cancer surgical waiting time and clinical outcome, but the answer is still controversial.8910
Extra-large hospitals are congested with the majority of patients, and this phenomenon might be more prominent in pancreatic cancer.11 However, clinically relevant data regarding the potential impact of surgical waiting times are lacking.
Therefore, we hypothesized that a short waiting time is correlated with better survival outcome than a long waiting time in patients who undergo pancreaticoduodenectomy (PD) for curative resection of pancreatic ductal adenocarcinoma (PDAC). The aim of this study is to investigate the correlation between surgical waiting time and overall survival and between surgical waiting time and cancer stage shifting in patients who underwent pancreaticoduodenectomy (PD) with resectable pancreatic ductal adenocarcinoma (PDAC), as well as to identify the major confounders that influence the association between them.
Go to : 
The present study is a retrospective cohort study of patients who underwent standard PD for pathologically confirmed PDAC at Asan Medical Center from January 2014 through December 2015, identified within an existing pancreatic cancer database of the Division of Hepatobiliary and Pancreatic Surgery. All patients were followed up with the same passive methods. All study procedures and ethics were approved by the institutional review boards of Asan Medical Center.
During the study period, 554 patients had surgical resection for PDAC at Asan Medical Center. Patients who had pancreatic body or tail cancer and who had neoadjuvant chemotherapy were excluded and these numbered 212 and 37, respectively. Among the remaining 322 patients, 166 patients underwent one or more adjacent organ resection and were excluded. Finally, 184 PD patients were included in this study. Details of the study population are reported in Fig. 1.
This study involved the historical follow-up of this cohort of individuals. Patients were dichotomized into those who underwent PD within the median of surgical waiting time and the others. Surgical waiting time was defined as the difference in date between initial diagnosis date and the date of operation. For assessing the initial diagnosis date, we reviewed and collected the date of initial abdominal computerized tomography (CT) or magnetic resonance imaging (MRI) for all eligible patients. Then, we assumed the surgical waiting time was the gap from initial imaging study, abdominal CT or MRI, to the operation date. The patient was unknown to the abstractor when she started reading each record. To maintain blinding, the passive follow-up of the patients with PD for PDAC was done by a different abstractor using an existing, computerized pancreatic cancer database and the inpatient and outpatient medical record system.
Standard PD was defined as conventional pancreaticoduodenectomy, pylorus-preserving pancreaticoduodenectomy (PPPD), or subtotal stomach-preserving pancreaticoduodenectomy (SSPPD), all performed by surgeons in our division.
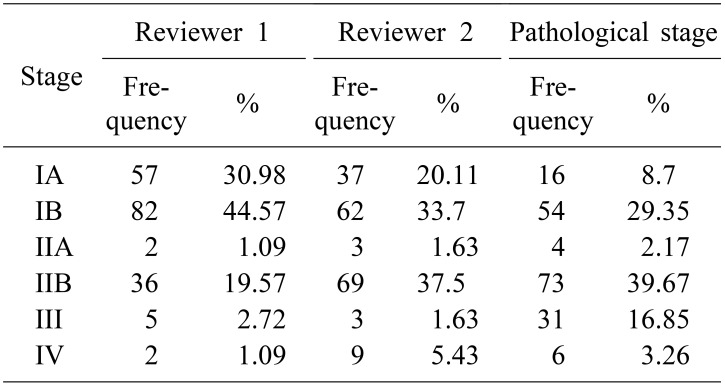
For the patients in this cohort, overall survival was defined as a primary outcome. Overall survival was commonly defined as the time between operation and death. We censored the patients who were alive at the end of the study (August 15, 2018) and those who could not be followed up further at the time of last contact. Secondary outcome was cancer stage shifting which is the difference between clinical stage at the imaging study and the final pathologic staging. All of these stages were decided according to the American Joint Committee on Cancer (AJCC) 8th Edition. Clinical stage was determined on initial CT scan by two independent, blinded surgeons, and pathologic stage was abstracted from the final pathology report. The data for pathologic staging were reviewed and collected from our medical record system. In addition, all the data related to demographics, surgery, pathology and oncology were considered as potential confounders or effect modifiers.
Statistical analysis was conducted using R 3.5.1. (R Foundation for Statistical Computing, Vienna, Austria). Categorical variables were reported as frequencies and continuous variables as median with range or mean with standard deviation, as appropriate. Categorical variables or proportions were compared using the chi-square test with continuity correction or Fisher's exact test. Continuous variables were compared using Student's t-test if normally distributedand the Wilcoxon rank-sum test otherwise. We obtained survival curves using the Kaplan-Meier method. To compare overall survival, we used log rank test as well as Cox proportional hazards models. Tests of significance were undertaken at the two-tailed alpha level of 0.05.
Go to : 
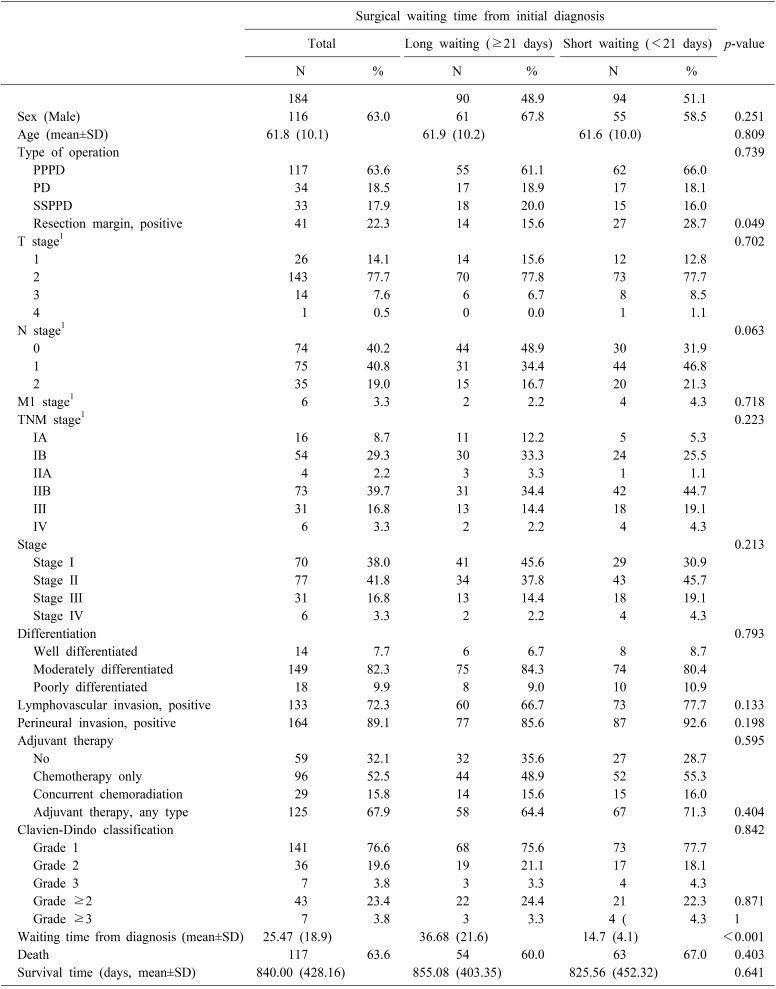
Among 184 patients, 116 (63.0%) were men; the mean age was 61.8±10.1 years at the time of operation. The median surgical waiting time between the date of initial imaging diagnosis and the date of operation in patients was 21 days (range, 6–149 days). The majority of operations were PPPD (n=117, 63.6%), and R1 resection was detected in 41 patients (22.3%). Forty-three patients (23.4%) experienced major morbidity greater than a grade 2 complication of the Clavien-Dindo classification. More than two-thirds of the patients received adjuvant therapy (n=125, 67.9%). In total, 117 patients (63.6%) died from any cause through August 15, 2018.
When we compared the two groups dichotomized by median surgical waiting time, the two groups were not highly imbalanced. Only one factor, the rate of R1 resection, was significantly higher in the short-waiting group (n=27, 28.7%) than the long-waiting group (n=14, 15.6%) (p=0.049). In addition, higher N stage was observed in the short-waiting group (N0/N1/N2=30 (31.9%)/44 (46.8%)/20 (21.3%)) than the long-waiting group (N0/N1/N2=44 (48.9%)/31 (34.4%)/15 (16.7%)), although the difference was only marginally significant (p=0.063). Their baseline characteristics are shown in Table 1.
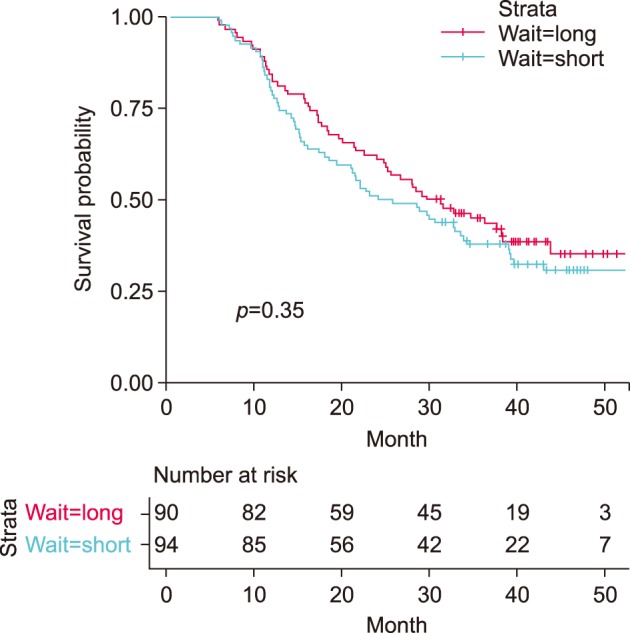
Fig. 2 shows the Kaplan-Meier curves of the two groups stratified by preoperative waiting time. Median overall survival did not differ between the long- and short-waiting group (30.4 vs 24.8 months, p=0.35). The hazard ratio of waiting time group estimated from univariate Cox proportional hazards model with overall survival as outcome was 0.84 (95% CI=0.58–1.21).
Table 2 reveals the clinical staging judged by two different surgeons and confirmative pathologic staging. The kappa statistic for clinical staging was 0.527 between two surgeons. To investigate the relationship between the waiting time and the cancer stage shifting, we compared the patients whose pathologic stage was higher than the clinical stage (upshifting) vs. those whose pathologic stage was lower than or equal to the clinical stage (downshifting). The difference in waiting time between the patients with upshifting vs. downshifting was not statistically significant. (p=0.112 and 0.114 by each of reviewers). The proportion of cancer stage shifting, upshifting or not, did not differ depending on waiting time group (p=0.811 and 0.225 by the respective reviewers) (Fig. 3).
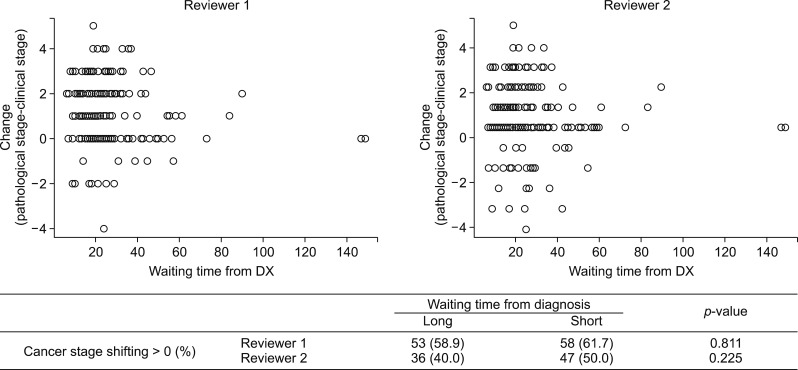
To investigate the possible trends in surgical waiting time and initial clinical stage, we performed analyses for the distribution of waiting time stratified by initial clinical staging and the correlation between those variables (Fig. 4). Short surgical waiting time was highly correlated with high initial clinical stage (Spearman correlation coefficients −0.201 (p=0.006) and −0.100 (p=0.175), computed by the respective reviewers).
Go to : 
The aim of this study was not to examine the results of PDAC treatment according to surgical waiting time but to study the possibility of improvement in overall survival of surgical resection for PDAC by earlier operation. However, in this study, overall survival was not associated with surgical waiting time in the patients who underwent PD for resectable PDAC. Likewise, cancer stage shifting was not increased in patients who waited longer for their operation. When we consider the exceptionally aggressive biological behavior of PDAC, the results of this study do not match with the well-known pathophysiology of PDAC.8912 By contrast, a few studies reported that a potential delay in curative surgery did not negatively affect the prognosis of most patients with PDAC.8131415 To assess this discrepancy, we tried to find what could affect the relationship between surgical waiting time and overall survival as a major confounder. The results showed that initial clinical stage had a negative correlation with surgical waiting time. Most of the previous studies involved surgical waiting time as a single factor that was assumed to be an objective value, but its negative correlation with initial clinical stage in our study means that surgical waiting time could be modified by the attending surgeon.89101415 The surgeon's consideration of preoperative clinical stage could affect waiting time, as could an uncertain diagnosis, preoperative biliary drainage, patient's general condition, and other factors.
In the era of centralization policy, much strong evidence supports the key role played by surgical volume in positively affecting the short-term and long-term outcomes.161718 When the patients are centralized to referral centers, the prolongation of surgical waiting time might be an inevitable consequence. The delay in treatment can affect patient survival.569 From this concept, some national health care systems recommend that the patients with an overt or suspected malignancy must be operated within a specific number of days from surgical consultation.819 However, one previous study revealed that more than 13% of surgery patients in Korea had experienced more than 30 days of waiting and that patients at high-volume hospitals waited longer for surgery than those in low- to medium-volume centers.10 Our study found 41 patients (22.3%) who waited on surgery more than 30 days, and this may reflect changes in health care service utilization with time.
Although this study could not prove the effect of surgical waiting time on survival, the patients with greater waiting time may have been more ill or presented with more advanced disease, which could require more aggressive perioperative intervention.91015 The negative correlation between preoperative clinical stage and waiting time was one of the confounders in this study, but a nationwide, large-scaled, population-based cohort study would be necessary to assess the survival effect of waiting time and the factors of waiting time, hopefully in the near future.
Our study had several limitations. First, nonrandomized observational studies should be interpreted with caution because of the potential for bias. Second, despite our adjustment for variables known to affect overall survival, we could not differentiate the confounding effect of variables related to the surgeon's decision on the timing of operation. Third, the reason for treatment delay was unknown in these cases. The patients with greater delay may have been more ill or presented with more advanced disease, which required greater preoperative intervention. On the other hand, the patients with suspicion of PDAC or with very early-stage cancer may have had a greater delay in surgical treatment. These factors could not be controlled in our study.
Despite its limitations, this study suggests that the confounding effect of initial clinical stage on the waiting time and overall survival should be considered when investigating the survival impact of waiting time and should be considered when using this result for formulating or revising national health policy on quality cancer care. Furthermore, evidence for maximum acceptable waiting time is not adequate, and further studies are needed. Close monitoring of the treatment delay with the impact of centralization is critical for evaluating policy at the national level.
Go to : 
ACKNOWLEDGEMENTS
This study was supported by a grant (2015-665) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
Go to : 
References
1. Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Park KM, et al. Chronologic changes in clinical and survival features of pancreatic ductal adenocarcinoma since 2000: a single-center experience with 2,029 patients. Surgery. 2018; 164:432–442. PMID: 29884479.

2. Hartwig W, Werner J, Jäger D, Debus J, Büchler MW. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 2013; 14:e476–e485. PMID: 24079875.

3. van Heek NT, Kuhlmann KF, Scholten RJ, de Castro SM, Busch OR, van Gulik TM, et al. Hospital volume and mortality after pancreatic resection: a systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005; 242:781–788. PMID: 16327488.
4. Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011; 49:1076–1081. PMID: 22002649.

5. Christensen ED, Harvald T, Jendresen M, Aggestrup S, Petterson G. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg. 1997; 12:880–884. PMID: 9489874.

6. Langenbach MR, Schmidt J, Neumann J, Zirngibl H. Delay in treatment of colorectal cancer: multifactorial problem. World J Surg. 2003; 27:304–308. PMID: 12607056.

7. Sainsbury R, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999; 353:1132–1135. PMID: 10209976.

8. Marchegiani G, Andrianello S, Perri G, Secchettin E, Maggino L, Malleo G, et al. Does the surgical waiting list affect pathological and survival outcome in resectable pancreatic ductal adenocarcinoma? HPB (Oxford). 2018; 20:411–417. PMID: 29191689.

9. Sanjeevi S, Ivanics T, Lundell L, Kartalis N, Andrén-Sandberg Å, Blomberg J, et al. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br J Surg. 2016; 103:267–275. PMID: 26572509.

10. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012; 23:2731–2737. PMID: 22553194.

11. Hong DP, Song J. The effective distribution system for the concentration of patients to extra-large hospitals. J Korean Surg Soc. 2011; 80:373–383. PMID: 22066063.

12. Marchegiani G, Andrianello S, Malleo G, De Gregorio L, Scarpa A, Mino-Kenudson M, et al. Does size matter in pancreatic cancer?: reappraisal of tumour dimension as a predictor of outcome beyond the TNM. Ann Surg. 2017; 266:142–148. PMID: 27322188.

13. Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008; 9:99–132. PMID: 18326920.
14. McLean SR, Karsanji D, Wilson J, Dixon E, Sutherland FR, Pasieka J, et al. The effect of wait times on oncological outcomes from periampullary adenocarcinomas. J Surg Oncol. 2013; 107:853–858. PMID: 23625192.

15. Healy GM, Redmond CE, Murphy S, Fleming H, Haughey A, Kavanagh R, et al. Preoperative CT in patients with surgically resectable pancreatic adenocarcinoma: does the time interval between CT and surgery affect survival? Abdom Radiol (NY). 2018; 43:620–628. PMID: 28695235.

16. Pieper D, Mathes T, Neugebauer E, Eikermann M. State of evidence on the relationship between high-volume hospitals and outcomes in surgery: a systematic review of systematic reviews. J Am Coll Surg. 2013; 216:1015–1025.e18. PMID: 23528183.

17. Hata T, Motoi F, Ishida M, Naitoh T, Katayose Y, Egawa S, et al. Effect of hospital volume on surgical outcomes after pancreaticoduodenectomy: a systematic review and meta-analysis. Ann Surg. 2016; 263:664–672. PMID: 26636243.
18. Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008; 95:357–362. PMID: 17933001.

19. BTS recommendations to respiratory physicians for organising the care of patients with lung cancer. The Lung Cancer Working Party of the British Thoracic Society Standards of Care Committee. Thorax. 1998; 53(Suppl 1):S1–S8.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download