Abstract
Backgrounds/Aims
The albumin-bilirubin (ALBI) score has been validated as a predictor of disease-free survival and overall survival in hepatocellular carcinoma (HCC). The purpose of this study was to assess the ALBI score as a risk factor for early recurrence (ER) after curative liver resection in HCC.
Methods
Patients who underwent liver resection with curative intent for HCC without previous treatment between January 2004 and December 2014 were included in this retrospective study. The utility of the ALBI score in predicting ER and late recurrence (LR) was evaluated.
Results
A total of 465 HCC patients were enrolled; multivariate analysis identified ALBI grade ≥2 (p=0.003) as a risk factor for ER, in addition to hepatitis B virus surface antigen (HBsAg)-positive status (p<0.001), tumor size ≥3.5cm (p≤0.001), lymph-vascular invasion (p=0.001), and the presence of satellite lesions (p=0.009). In subgroup analysis for ALBI grade 1, Model for End-stage Liver Disease score >9 (p=0.046), HBsAg positive status (p=0.004), tumor size ≥3.5 cm (p<0.001), lymph-vascular invasion (p=0.001), presence of satellite lesions (p=0.002), and poor tumor differentiation (p=0.007) were independent risk factors for ER; however, in subgroup analysis for ALBI grade 2, no significant associations with ER were found. Kaplan-Meier curve analysis showed that long-term survival in HCC with ER was significantly shorter than in patients with LR.
Hepatocellular carcinoma (HCC) is common, and is one of the leading causes of death worldwide.1 Liver resection is standard treatment for HCC, followed by liver transplantation, but is a major cause of death in long term follow-up due to recurrence, even after liver resection.234 The overall recurrence rate is reportedly 54% to 63% after HCC treatment.56 Other studies have shown an early recurrence rate of 38%7 less than a year after radical hepatectomy, and it is known that tumor stage, presence of microsatellites, microvascular invasion, liver cirrhosis, multinodularity, hepatitis activity, and alpha-fetoprotein (AFP) level are associated with early recurrence.891011 It is very important to determine the risk factors for early recurrence and to diagnose early postoperative recurrence with strict follow-up because transarterial chemoembolization (TACE), radiofrequency ablation (RFA), or reoperation can increase survival duration.12
The albumin-bilirubin (ALBI) score has been highlighted as a predictor of postoperative hepatic failure and long-term survival after hepatic resection, and is a new tool for the assessment of liver failure and function.12 According to another study, ALBI grade was associated with significant differences in disease-free survival after hepatectomy for HCC.13
We hypothesized a relationship between ALBI score and early recurrence (ER). To date, no study has determined whether ALBI grade is associated with recurrence within a year (we defined this as ER). Therefore, this study aimed to investigate the usefulness of ALBI score for relapse within a year.
Between January 2004 and December 2014, all consecutive patients who underwent liver resection with curative intent for HCC at the Hwasun Chonnam National University Hospital were considered for this retrospective study. The inclusion criteria were: initial liver resection with curative intent performed at the authors' center; no treatment for HCC before liver resection; and no other simultaneous malignancies.
We defined ER as recurrence within a year after curative liver resection for HCC, based on a study by Tung-Ping Poon et al.12 Diagnosis of HCC was based on histological evidence after surgery. Major liver resection was defined as resection of at least three Couinaud liver segments.14 In pathologic reports, vascular invasion included gross as well as microscopic invasion of vessels. Microscopic vascular invasion is defined by tumor within a vascular space lined by endothelium, identified only on microscopy in the capsule or noncapsular fibrous septa, or liver tissue surrounding the tumor. The ALBI score is a validated formula used for rigorous statistical analysis of HCC patients, based on bilirubin and albumin levels.15 In this study, the ALBI score was calculated using the following formula with preoperative laboratory analysis: 0.66× log10 (total bilirubin [µmol/l]) −0.085 (albumin[g/l])
The ALBI score was stratified as grade 1 (−2.60 or less), grade 2 (−2.59 to −1.39), or grade 3 (greater than −1.39).
All patients were followed up at 1 month after liver resection, followed by every 3 months in the first year and every 3-6 months in subsequent years. Routine tests such as serum AFP levels, serum biochemistry, chest X-ray, abdominal ultrasound, and abdominal computed tomography or magnetic resonance imaging were performed at every follow-up. Patients with relapses were treated with liver resection, percutaneous ethanol injection, RFA, TACE, or sorafenib, depending on liver functional status, extent of disease, and overall health and economic status.
Statistical analysis was performed using SPSS® version 22.0 (IBM, Armonk, NY, USA). Student's t-test, the Mann-Whitney U test, and the χ2 test were used to compare continuous and categorical variables, as appropriate. Multivariate analysis was performed using a logistic regression model to identify independent predictors of early recurrence. The Kaplan-Meier method was used to estimate overall survival. Data are expressed as number of patients, ratio (%), or odds ratio (OR) and 95% confidence interval (CI), as indicated. A p-value <0.05 was considered statistically significant.
Of 579 patients who underwent hepatic resection during the study period, 114 were excluded for the following reasons: 30 received treatment for HCC before liver resection, 21 underwent intraoperative RFA, 2 had surgery with non-curative intent, 4 had other malignant tumors, 13 died during the perioperative period, and 44 were lost to follow-up. The remaining 465 patients were enrolled in the study (Fig. 1).
Patient demographics and clinicopathological features are listed in Table 1. Among the 465 patients, 398 were male (85.6%) and 67 were female (14.4%). The mean patient age was 59.2 years. Overall, 290 patients (62.4%) had recurrence after liver resection with curative intent. ER was observed in 140 patients (30.1%) in the first year after liver resection and 150 patients (32.3%) had recurrence after the first year (late recurrence, LR). Recurrence was not observed in 175 patients (37.6%). The majority (98.1%) had Child-Pugh (CP) grade A disease and the remaining 1.9% had CP grade B disease. The Model for End-stage Liver Disease (MELD) score was ≤ 9 in 94.8% of patients, between 10 and 19 in 4.9%, and between 30 and 39 in 0.2%. The preoperative ALBI score classified 78.5% of the patients as grade 1, 21.1% as grade 2, and 0.4% as grade 3.
Univariate analysis to identify prognostic factors affecting ER showed that AFP ≥40 (p=0.038, OR: 1.53, 95% CI: 1.02–2.30), preoperative ALBI grade ≥2 (p<0.001, OR: 2.46, 95% CI: 1.55–3.88), MELD score >9 (p=0.034, OR: 2.45,95% CI: 1.06–5.64), hepatitis B virus surface antigen (HBsAg)-positive status (p=0.014, OR: 1.72, 95% CI: 1.12–2.68), presence of multiple tumors (p=0.007, OR: 2.16, 95% CI: 1.22–3.80), tumor size ≥3.5 cm (p<0.001, OR: 3.34, 95% CI: 2.16–5.29), vascular invasion (p=0.001, OR: 2.25, 95% CI: 1.42–3.63), lymph-vascular invasion (p<0.001, OR: 3.72, 95% CI: 2.30–6.04), intrahepatic metastasis (p<0.008, OR: 8.50, 95% CI: 2.02–57.55), multicentricity (p<0.006, OR: 2.43, 95% CI: 1.28–4.60), presence of satellite lesions (p<0.001, OR: 2.91, 95% CI: 1.80–4.72), tumor necrosis (p<0.001, OR: 2.86, 95% CI: 1.90–4.35), and tumor differentiation (p=0.002, OR: 1.86, 95% CI: 1.25–2.78) were significant risk factors (Table 2). After these variables were included in multivariate analysis, ALBI grade ≥2 (p<0.003, OR: 2.60, 95% CI: 1.55–4.38), HBsAg-positive status (p=0.001, OR: 2.37, 95% CI: 1.45–3.96), tumor size ≥3.5 cm (p≤0.001, OR: 2.81, 95% CI: 1.72–4.66), lymph-vascular invasion (p=0.001, OR: 2.44, 95% CI: 1.41–4.22), and presence of satellite lesions (p=0.009, OR: 2.11, 95% CI: 1.21–3.67) were found to be independently predictive of ER.
Univariate analysis to identify prognostic factors affecting LR showed that anti-hepatitis C virus (HCV)-positive status (p<0.001, OR: 4.30, 95% CI: 2.34–8.12) and tumor size ≥3.5 cm (p=0.010, OR: 0.60, 95% CI: 0.40–0.88) were significant risk factors. On multivariate analysis, anti-HCV-positive status (p<0.001 OR: 0.63, 95% CI: 0.42–0.93) and tumor size ≥3.5 cm (p=0.022, OR: 0.89, 95% CI: 0.79–0.98) were significant risk factors (Table 3).
We performed subgroup analysis by preoperative ALBI grade 1 and 2 after excluding 2 patients of ALBI grade 3 (Table 4). In the ALBI grade 1 subgroup, MELD score >9 (p=0.012, OR: 6.04, 95% CI: 1.56–29.11), HBsAg-positive status (p=0.016, OR: 1.93, 95% CI: 1.14–3.36), presence of multiple tumors (p=0.006, OR: 2.47, 95% CI: 1.28–4.72), tumor size ≥3.5 cm (p<0.001, OR: 3.57, 95% CI: 2.14–6.16), vascular invasion (p=0.003, OR: 2.28, 95% CI: 1.35–3.97), lymph-vascular invasion (p<0.001, OR: 4.33, 95% CI: 2.47–7.64), bile duct invasion (p=0.042, OR: 5.93, 95% CI: 1.14–43.32), intrahepatic metastasis (p=0.014, OR: 15.06, 95% CI: 2.39–290.49), presence of satellite lesions (p<0.001, OR: 3.51, 95% CI: 2.00–6.17), tumor necrosis (p<0.001, OR: 3.19, 95% CI: 1.96–5.29), and tumor differentiation (p<0.001, OR: 2.38, 95% CI: 1.48–3.87) were associated with a high risk of ER on univariate analysis; MELD score >9 (p=0.046, OR: 5.80, 95% CI: 1.08–36.29), HBsAg-positive status (p=0.004, OR: 2.50, 95% CI: 1.36–4.75), tumor size ≥3.5 cm (p<0.001, OR: 3.47, 95% CI: 1.96–6.32), lymph-vascular invasion (p=0.001, OR: 2.90, 95% CI: 1.52–5.54), presence of satellite lesions (p=0.002, OR: 2.76, 95% CI: 1.43–5.30), and tumor differentiation (p=0.007, OR: 2.10, 95% CI: 1.23–3.62) were found to be independent risk factors in multivariate analysis. In the ALBI grade 2 subgroup, no risk factors were associated with ER (Table 5).
The Kaplan-Meier curve showed that significantly lower overall survival in ER patients than non-ER patients (Fig. 2).
In this study, the risk factors for ER after curative liver resection in HCC patients were AFP ≥40, preoperative ALBI grade ≥2, HBsAg-positive status, presence of multiple tumors, large tumor size, vascular invasion, lymph-vascular invasion, intrahepatic metastasis, multicentricity, presence of satellite lesions, tumor necrosis, and poor tumor differentiation, consistent with the findings in previous studies. The risk factors reported in previous studies suggest that intrahepatic metastases are the main cause of early intrahepatic recurrence.416 In this study, ALBI score was found to be associated with ER, which was not reported in previous studies.
ALBI score has been identified as one of the best indicators of liver function. Most studies have shown that the ALBI score may be a better predictor of liver function than the CP score, MELD score, and ICGR15 and is therefore more useful as a predictor of postoperative hepatic failure.17 Moreover, several studies have found that ALBI is associated with prognostic factors for disease-free survival and overall survival.13 Kanda et al.18 reported that ALBI grade 2 patients were more likely to have shorter disease-specific and disease-free survival after radical gastrectomy, compared with that for ALBI grade 1 patients. Additionally multivariable analysis identified ALBI grade 2 as an independent prognostic factor for disease-free survival.
We performed subgroup analysis based on the assumption that liver function is associated with ER. In ALBI grade 1 patients, the risk factors for ER were tumor size ≥3.5 cm, venous/lymphatic invasion, presence of satellite lesions, and poor tumor differentiation; however, grade 2 patients showed no associations with ER. It can be assumed that ER is mainly affected by histological factors in ALBI grade 1 patients, which means liver function is favorable. However in ALBI grade 2 patients, no risk factors were associated with ER. According to these results, liver function impairment is mainly associated with ER when liver function is not favorable. Lise et al.3 reported that CP class was an independent prognostic factor for disease-free survival and overall survival in multivariate analysis, while liver function impairment may be associated with recurrence of HCC. Hirokawa et al.19 reported that ER after curative hepatectomy in HCC patients was associated with ICGR15 >16%, and that recurrence patterns and risk factors vary by liver function status. These results suggest that liver function may be associated with ER.
Our study has some limitations. First, this study was conducted at a single institution, and the retrospective study design might have resulted in bias. Second, the most common cause of HCC in this study was hepatitis B virus, but HCC in Western countries is associated with other causes such as hepatitis C virus infection. Therefore, more studies are required according to etiological populations. Despite these limitations, this study showed that ALBI grade was associated with ER after curative liver resection for HCC and could be useful as a preoperative predictor of ER. The ALBI grade can also be used to determine the likelihood of resection or transplantation because liver function can be assessed.
In conclusion, preoperative ALBI grade ≥2 is a preoperative risk factor for ER, along with other well-known factors, and requires more thorough follow-up. ALBI grade may be useful in determining appropriate management according to liver function when recurrence develops.
Figures and Tables
Fig. 1
Flow chart of patient selection procedures in this study. HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; IORFA, intraoperative radiofrequency ablation.

Fig. 2
Overall survival of early recurrence (≤1 year) after curative hepatectomy in hepatocellular carcinoma patients.

Table 1
Baseline clinicopathological characteristics

AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein; ICGR15, indocyanine green retention test at 15 minutes; ALBI, albumin-bilirubin; MELD, model for end-stage liver disease; HBsAg, hepatitis B surface antigen; Anti-HCV, Anti-hepatitis C virus; POD7, Postoperative day 7
Table 2
Univariate and multivariate analysis of risk factors for early recurrence (≤1 year) after curative liver resection in HCC patients
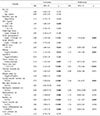
Table 3
Univariate and multivariate analysis of risk factors for late recurrence (>1 year) after curative liver resection in HCC patients

Table 4
Univariate and multivariate analysis of risk factors for early recurrence (≤1 year) in ALBI grade 1 after curative liver resection in HCC patients

References
1. Park JH, Koh KC, Choi MS, Lee JH, Yoo BC, Paik SW, et al. Analysis of risk factors associated with early multinodular recurrences after hepatic resection for hepatocellular carcinoma. Am J Surg. 2006; 192:29–33.

2. Chen MF, Hwang TL, Jeng LB, Wang CS, Jan YY, Chen SC. Postoperative recurrence of hepatocellular carcinoma. Two hundred five consecutive patients who underwent hepatic resection in 15 years. Arch Surg. 1994; 129:738–742.
3. Lise M, Bacchetti S, Da Pian P, Nitti D, Pilati PL, Pigato P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma: results in a series of 100 Italian patients. Cancer. 1998; 82:1028–1036.
4. Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002; 235:373–382.
5. Lee SY, Konstantinidis IT, Eaton AA, Gönen M, Kingham TP, D'Angelica MI, et al. Predicting recurrence patterns after resection of hepatocellular cancer. HPB (Oxford). 2014; 16:943–953.

6. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015; 261:947–955.
7. Shah SA, Greig PD, Gallinger S, Cattral MS, Dixon E, Kim RD, et al. Factors associated with early recurrence after resection for hepatocellular carcinoma and outcomes. J Am Coll Surg. 2006; 202:275–283.

8. Cheng Z, Yang P, Qu S, Zhou J, Yang J, Yang X, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. . HPB (Oxford). 2015; 17:422–427.

9. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003; 38:200–207.

10. Li T, Fan J, Qin LX, Zhou J, Sun HC, Qiu SJ, et al. Risk factors, prognosis, and management of early and late intrahepatic recurrence after resection of primary clear cell carcinoma of the liver. Ann Surg Oncol. 2011; 18:1955–1963.

11. Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009; 51:890–897.

12. Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000; 232:10–24.

13. Harimoto N, Yoshizumi T, Sakata K, Nagatsu A, Motomura T, Itoh S, et al. Prognostic significance of combined albumin-bilirubin and tumor-node-metastasis staging system in patients who underwent hepatic resection for hepatocellular carcinoma. Hepatol Res. 2017; 47:1289–1298.

14. Pol B, Campan P, Hardwigsen J, Botti G, Pons J, Le Treut YP. Morbidity of major hepatic resections: a 100-case prospective study. Eur J Surg. 1999; 165:446–453.
15. Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015; 33:550–558.

16. Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Recurrence of hepatocellular carcinoma after surgery. Br J Surg. 1996; 83:1219–1222.

17. Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg. 2016; 103:725–734.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download