This article has been
cited by other articles in ScienceCentral.
Abstract
Portal hypertension induces congestion of the liver and spleen, thus any capsular or parenchymal injury to these organs can produce intractable bleeding which generally is not easily controlled. To cope with intractable bleeding such as being encountered during liver transplantation, we developed an infiltrating hemostasis technique as a conceptual shift from conventional application methods, in which fibrin glue is locally injected into the bleeding area on the liver or spleen. This technique, which uses a fibrin glue kit (2 ml kit; Greenplast, Green Cross, Seoul, Korea), consists of inserting the needle 0.5-1 cm deep at the bleeding point, forcefully injecting 1 ml of fibrin glue contained in the fibrin glue kit, and then slowly withdrawing the needle with continuous forceful injection of the remaining 1 ml of fibrin glue. We have successfully performed this procedure in 6 cases of living donor liver transplantation and in 2 cases of non-transplant resection of the cirrhotic livers with hepatocellular carcinoma. This technique was also successfully applied to one liver transplant recipient in which intractable bleeding occurred from a small laceration at the spleen. Our fibrin glue-infiltrating hemostasis would be indicated to intractable bleeding from the hepatic or splenic cut surface. In such a situation, it would be applicable as a second-line rescue method for hemostasis.
Go to :

Keywords: Hemostasis, Fibrin glue, Bleeding, Liver transplantation
INTRODUCTION
Portal hypertension induces congestion in the liver and spleen, thus any capsular or parenchymal injury to these organs can cause intractable bleeding which is usually not easily controlled. During living donor liver transplantation (LDLT) using a modified right lobe graft, bleeding from the cut surface of the graft liver is occasionally difficult to control because some hepatic parenchyma does not drain well into the reconstructed middle hepatic vein (MHV) outflow pathway.
12 In such a situation, any deep suturing at the cut surface may produce additional damage making control of bleeding even more difficult. If the splenic capsule is torn during manipulation of the liver, control of capsule bleeding through conventional hemostasis methods may be difficult due to splenic congestion from portal hypertension. To cope with such intractable bleeding during liver transplantation, we developed the infiltrating hemostasis technique in which fibrin glue is locally injected into the intractable bleeding area of the liver or spleen.
Go to :

MECHANISMS AND TECHNIQUES OF FIBRIN GLUE-INFILTRATING HEMOSTASIS
One of the most common causes of bleeding of the cut surface of liver grafts is tearing of MHV branches which usually occurs during bench work for vessel graft interposition. Since the parenchyma at the hemiliver cut surface is soft, without supporting connective tissues, and portal hypertension induces parenchymal congestion, direct suturing for bleeding control is more difficult than in the usual non-transplant hepatectomy cases. Furthermore, hemostasis via electrocautery or argon beam coagulation is not applicable to the bleeding site around the MHV branch reconstruction because it can melt the monofilament suture materials.
In such intractable bleeding, the conventional methods for hemostasis include initial suturing with 5-0 or 6-0 monofilament with or without application of pledget material, topical application of various hemostasis materials, mechanical compression, and finally deep suturing with sacrifice of MHV branch reconstruction. Since the normal hepatic parenchyma is soft in nature, and iatrogenic damage makes it more friable, it is difficult to achieve complete hemostasis through mechanical or topical therapy.
Accordingly, we developed a new method of hemostasis to control the bleeding beneath the bleeding surface. Most commercial products of fibrin glue are designed to be spread over a dried surface, and their adhesive strength is not high enough to induce hemostasis at a wet bleeding surface. Thus, we infiltrated the fibrin glue directly into the hepatic parenchyma, at a 0.5-1 cm depth from the bleeding surface (
Fig. 1). This procedure made the drops of fibrin glue scatter in the hepatic parenchyma, causing mechanical compression of the small bleeding vessels and instant cessation of bleeding at the liver surface. Cross-sectional examination of the fibrin glue-infiltrated area shows that the fibrin glue drops were dispersed only in the small area of hepatic parenchyma and did not enter into the blood vessels (
Fig. 2). The technique, which uses a fibrin glue kit (2 ml; Greenplast, Green Cross, Seoul, Korea), consists of insertion of the needle 0.5-1 cm deep at the bleeding point, forceful injection of 1 ml of the fibrin glue, and then slow withdrawal of the needle with continuous forceful injection of the remaining 1 ml of fibrin glue. We performed this procedure in 6 cases of LDLT and 2 cases of non-transplant resection of cirrhotic livers with hepatocellular carcinoma. Topical injection of 1 or 2 kits of fibrin glue led to instant, complete hemostasis in all 8 cases.
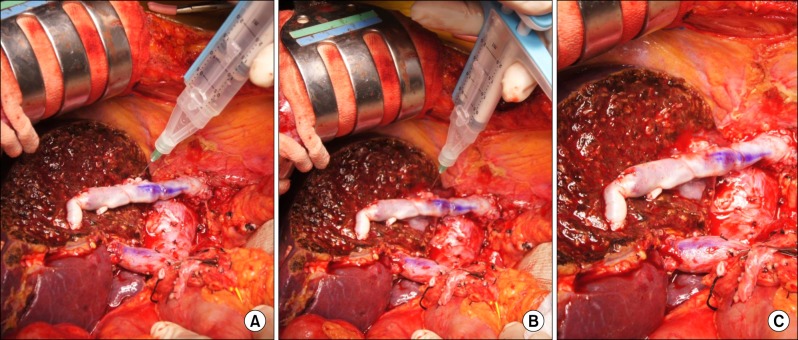 | Fig. 1Operative photographs of fibrin glue-infiltrating hemostasis procedure: (A) fibrin glue needle insertion into the liver parenchyma by 0.5-1 cm in depth; (B) slow withdrawal of the needle with continuation of injection; and (C) completion of hemostasis procedure.
|
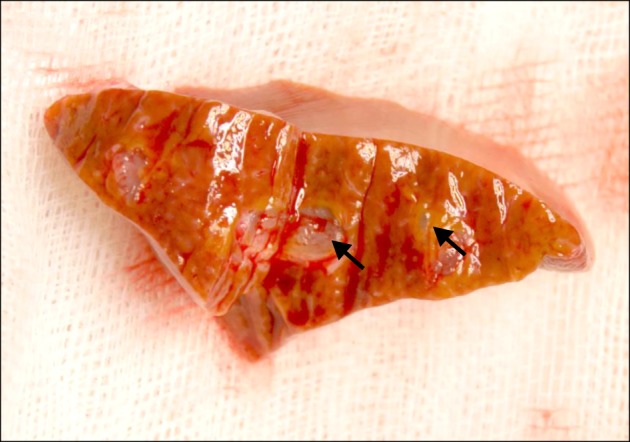 | Fig. 2Photograph of the cross-section of the fibrin glue-injected liver parenchyma showing dispersed fibrin glue drops (arrows).
|
This technique was also applied to one LDLT recipient who had a small laceration on the spleen. A 1 cm-long tear on the anterior-inferior surface of the spleen probably occurred during mobilization of the liver. Local control with topical application of various hemostatic materials and compression was attempted, but was unsuccessful due to portal hypertension. Active oozing continued even after splenic artery ligation. We injected 2 kits of fibrin glue, and the bleeding instantly stopped. However, after 1 hour, we found minute oozing. Additional injection of 2 kits of fibrin glue at the same area led to complete hemostasis. Follow-up computed tomography at 5 days showed the shallow intrasplenic infiltration of fibrin glue drops (
Fig. 3).
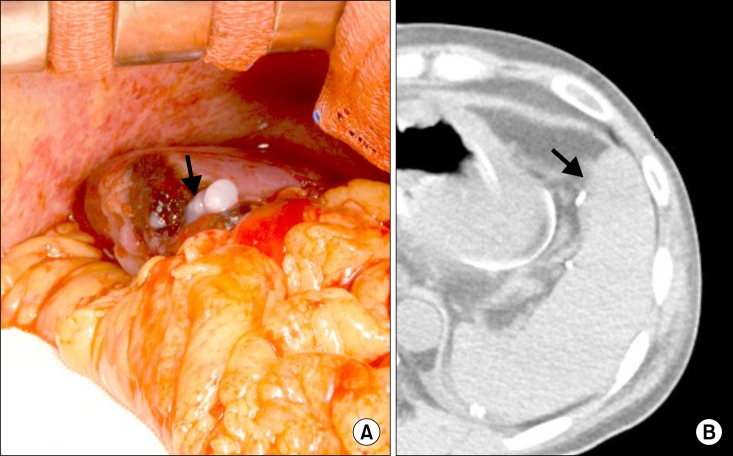 | Fig. 3A case of splenic laceration in a patient with portal hypertension. (A) fibrin glue-infiltrating hemostasis (arrow) was applied; and (B) shallow intrasplenic infiltration of fibrin glue drops (arrow) is barely visualized in a follow-up non-enhanced computed tomography taken at posttransplant 5 days.
|
Go to :

DISCUSSION
Fibrin glues or sealants are a group of topical hemostatic products that mimic the final stages of the blood coagulation process. Fibrin sealants are two-component products, containing thrombin and fibrinogen. When these are mixed together during application, thrombin cleaves the fibrinogen to monomers, which polymerize to form a fibrin gel.
34 Fibrin sealants come in two-component vials to form glue, but they can also be carried on a matrix, the so-called carrier-bound fibrin sealants. Carrier-bound sealants are available in two forms, a solid form, consisting of a collagen fleece coated with a dry form of fibrinogen and thrombin, and a liquid form containing thrombin and gelatin or thrombin and collagen.
5 It is one of the commonly used materials for liver surgery because it can be effective as an adjunct to achieve hemostasis during liver resections.
However, there is lack of evidence on the efficacy of fibrin sealants in reducing postoperative resection surface-related complications. Therefore, routine use of fibrin sealants in liver surgery has not been recommended.
5 A recent meta-analysis study including 10 randomized controlled trials with 1,225 patients indicated that there is no solid evidence that the routine use of fibrin sealants reduces the incidence of postoperative hemorrhage or bile leakage compared with other treatments. The use of fibrin sealants may decrease the time to hemostasis, but this does not translate to improved perioperative outcomes.
6
To date, fibrin glue has been used as a topical application which limits its adhesive strength. Our concept of fibrin glue-infiltrating hemostasis is a kind of anchoring within the hepatic or splenic parenchyma, by which drops of intraparenchymal fibrin glue mechanically compress the surrounding minute bleeding vessels. Thus our method is a conceptual shift from conventional application methods. The infiltration of fibrin glue superficially penetrates into the hepatic or splenic parenchyma and drops are formed within a few seconds, thus no systemic effect or penetration into vessels occurs. Therefore its safety level is nearly similar to that of the usual surface spray method.
Our fibrin glue-infiltrating hemostasis would be indicated to intractable bleeding from the hepatic or splenic cut surface. In such a situation, it would be applicable as a second-line rescue method for hemostasis.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download