Abstract
Backgrounds/Aims
We investigated patients' clinical and radiological data to determine preoperative factors that predict cholesterol gallbladder (GB) polyps of large size, which can be helpful for decision on further diagnostic tools.
Methods
In this study, we retrospectively analyzed 126 patients who underwent laparoscopic cholecystectomy for GB polyps >10 mm diagnosed preoperatively by abdominal ultrasonography between February 2002 and February 2016 in Department of Surgery, Sanggye Paik Hospital. Patients were divided into non-cholesterol polyps group and cholesterol polyps group, based on the postoperative pathologic diagnosis. Clinical and radiological data, such as gender, age, body weight, height, body mass index (BMI), laboratory findings, size, number and shape of the polypoid lesions, and presence of the concurrent GB stone were compared between the two groups.
Results
Of the 126 cases, 73 had cholesterol polyps (57.9%) and 53 cases were non-cholesterol polyps (42.1%). The younger age (<48.5 years), size of polyp <13.25 mm and multiple polyps were independent predictive variables for cholesterol polyps, with odd ratios (OR) of 2.352 (p=0.045), 5.429 (p<0.001) and 0.472 (p<0.001), respectively.
Conclusions
Age, size and polyp number were used to predict cholesterol GB polyp among polypoid lesions of the gallbladder >10 mm. For cases in which these factors are not applicable, it is strongly recommended to evaluate further diagnostic tools, such as computed tomography, endoscopic ultrasonography and tumor markers.
A polypoid lesion of the gallbladder (PLG) is defined as any elevated lesion of the mucosal surface of the gallbladder wall.12 Recent advances in radiologic tools such as ultrasonography (USG) and computed tomography (CT) have contributed to the early detection of gallbladder (GB) cancer. Eighty percent of GB cancers are characteristically detected as polypoid lesions, and differential diagnosis can be carried out by the pathologic examination alone.3 Distinguishing between benign and malignant polyps is important to facilitate early diagnosis and treatment, because untreated malignant GB polyps could cause a poor prognosis.45
Currently, the treatment of a PLG is in accordance with the “Gallbladder polyp practice recommendation” of the Korean Association of Hepato-Biliary-Pancreatic Surgery (KAHBPS). The recommended risk factors for distinguishing malignancy from PLGs include size, number and shape of polyps, interval change of size, age, and presence of the concurrent GB stone. These factors can only be found through GB specimen from surgical resection with initial diagnostic tool of USG. According to the “Gallbladder polyp practice recommendation” of KAHBPS polyp size of >10 mm or irregular GB wall thickening in USG are suggestive of malignancy. Among patients with suspicious malignant GB polyp in USG, further evaluations, such as CT, endoscopic ultrasonography (EUS) and tumor markers may be required.
If cholesterol polyps can be differentiated from non-cholesterol polyps in large size polyps, it would be helpful in decision-making for further pre-operative evaluations. Therefore, we retrospectively evaluated clinical and radiologic data that would distinguish cholesterol polyps from non-cholesterol polyps.
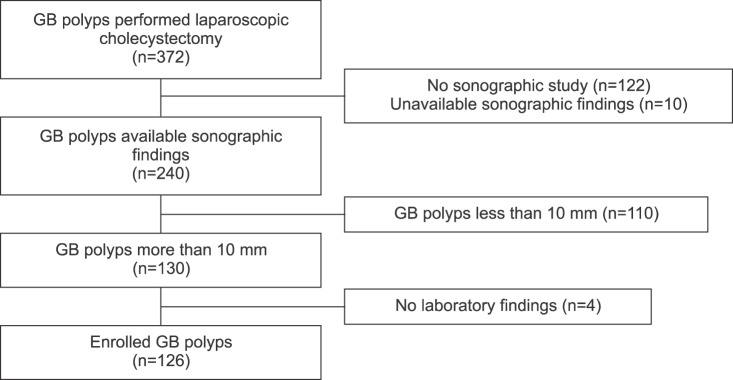
We performed retrospective analysis on the consecutively enrolled patients who were diagnosed with a PLG >10 mm by preoperative abdominal USG between February 2002 and February 2016 in Department of Surgery, Sanggye Paik Hospital. During the study period, a total of 372 cases of PLGs underwent laparoscopic cholecystectomy. We excluded 246 cases, including 12 cases with unavailable sonographic finding, 4 cases with insufficient laboratory results, 110 cases with small polyps (<10 mm), and 122 cases in which preoperative abdominal USG was not performed. Finally, 126 cases were analyzed in this study (Fig. 1). Patients were divided into two groups, including non-cholesterol polyps group and cholesterol polyps group based on postoperative pathologic diagnosis.
Comparative analysis was conducted on clinical features such as gender, age, body weight, BMI, laboratory findings, size (maximal diameter measured by abdominal USG), the number and shape of PLGs, and presence of the concurrent GB stone. All statistical analyses were carried out using MedCalc statistical software ver. 15.5 (MedCalc Software, Ostend, Belgium; https://www.medcalc.org; 2015). Results were reported as the mean±standard deviation. For statistical analysis, a Chi-square, t-test and Mann-Whitney U test were used, and multivariate binary logistic regression analysis was performed to determine the significance of the various predictive variables that were significant on univariate analysis. Receiver operating characteristic (ROC) curve analysis was also performed for correlation analysis. p-values of <0.05 were considered as significant.
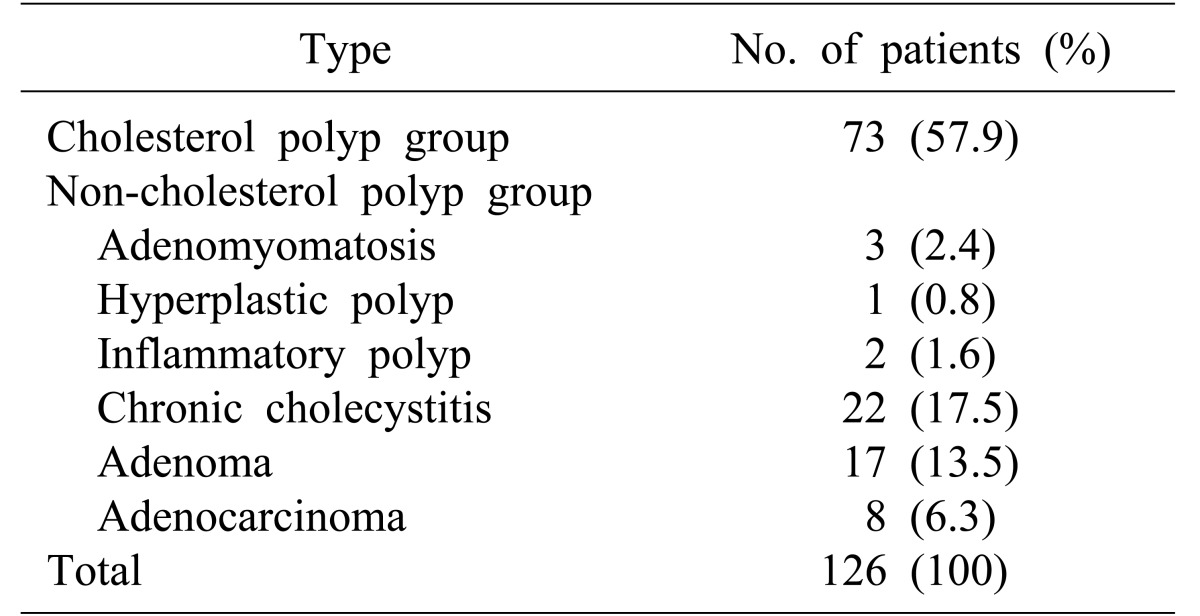
Of the 126 patients included in the study, 73 had cholesterol polyps (57.9%) and 53 cases were non-cholesterol polyps (42.1%). Among the 53 non-cholesterol polyps, 22 cases (17.5%) were chronic cholecystitis, 17 cases (13.5%) were adenoma, 8 cases (6.3%) were adenocarcinoma, 3 cases (2.4%) were adenomyomatosis, 2 cases (1.6%) were inflammatory polyps and 1 case (0.8%) was hyperplastic polyp (Table 1).
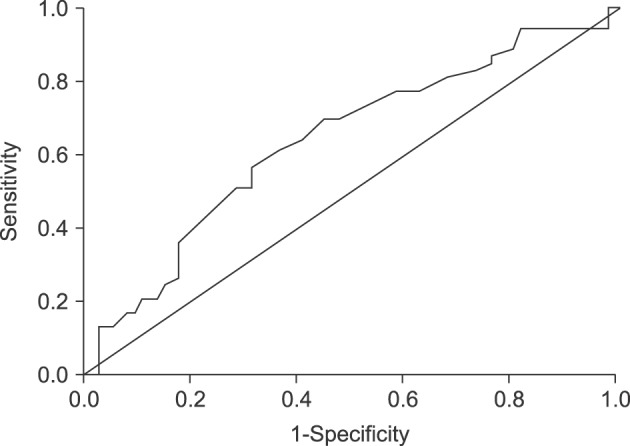
Of the 126 patients, 66 were female and 60 were male. The percentage of cholesterol polyps were higher in both genders (56.7% in female and 59.1% in male), and there were no statistically significant differences in the gender ratio between the cholesterol polyp group (M:F=1:1.15) and the non-cholesterol polyp group (M:F=1:1.04). The mean ages were 45.15±11.89 years for the cholesterol group and 50.68±12.37 years for the non-cholesterol polyp group, without significance between the two groups (p=0.013) (Table 2). The mean BMI was 24.15±3.09 kg/m2 in the cholesterol polyp group and 24.12±3.26 kg/m2 in the non-cholesterol polyp group, without significance between the two groups (p=0.950). The serum cholesterol level was 194.48±33.89 mg/dl in the cholesterol polyp group and 189.90±37.34 mg/dl in the non-cholesterol polyp group, without significant difference between the two groups (p=0.475) (Table 2). For the detailed analysis, the age was divided into 2 categories by use of receiver-operator characteristic (ROC) curves. At a cutoff value of 48.5 years, the area under the ROC curve (AUC) showed the highest sensitivity and specificity of 60.4%, 64.4%, respectively (Fig. 2).
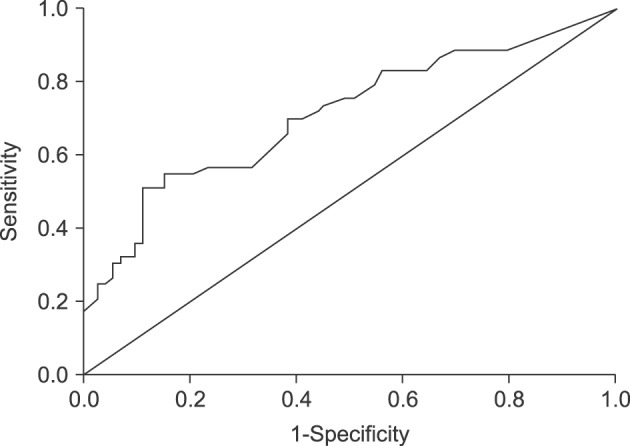
Sonographic findings indicated that the mean maximal diameters of the polyps were 11.85±2.34 mm for the cholesterol polyp and 15.70±6.41 mm for the non-cholesterol polyp group, with significant difference between the two groups (p<0.001). In addition, multiple lesions revealed significant correlation with cholesterol polyps (p<0.001). However, the shape of the polypoid lesions (p=0.132) and presence of the concurrent GB stone (p=0.491) were not significantly correlated with cholesterol polyps (Table 3). For the detailed analysis, maximal diameter was divided into 2 categories by use of ROC curves. At a cutoff value of 13.25 mm, the AUC showed the highest sensitivity and specificity of 54.7% and 84.9%, respectively (Fig. 3).
The univariate analysis revealed several important predictive clinical and radiologic values such as an age <48.5 years, sonographic size <13.25 mm, and multiple lesions. On multivariate analysis, younger age (<48.5 years), polyp size (<13.25 mm) and multiple lesions were independent predictive factors for cholesterol polyps with odd ratios (OR) of 2.352 (p=0.045), 5.429 (p <0.001) and 0.472, respectively (p<0.001) (Table 4).
GB polyps represent a wide spectrum of abnormalities from pseudo-lesions to GB cancer. GB polyps consist of true neoplasms, such as benign adenomas and adenocarcinomas, and non-neoplastic polyps, such as cholesterol polyps, inflammatory polyps, and adenomyomatous hyperplasia. Of the benign GB polyps, the cholesterol polyp is reported as the most common type.6789 Distinguishing between benign polyps and malignant polyps is very important because untreated malignant GB polyps could cause poor prognosis.
Currently, risk factors of malignant polyps such as size, number and shape of polyps, interval change of size, and presence of the concurrent GB stone can be found only through GB specimen obtained from surgical resection with pre-operative diagnostic imaging including abdominal USG, CT, EUS, magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT). Preoperative diagnostic laboratory findings serve as tumor markers. Abdominal USG is considered the best available exam for diagnosing GB polyps, not only because of its accessibility and low cost, but also because of high sensitivity and specificity.1011 The polyps can be located, counted, and measured with ultrasound, and the three layers of the GB wall and any abnormalities can be viewed.12 The polyps appear as fixed, hyperechoic material protruding in to the lumen of the gallbladder, with or without an acoustic shadow. Abdominal USG has higher sensitivity for diagnosis of GB polyps than oral cholecystography as well as CT and can distinguish a cholesterol polyp from an adenoma or an adenocarcinoma. However, the distinction is difficult to make, and the status of polyps as benign or malignant cannot be determined with abdominal USG alone.12
EUS is more accurate than abdominal USG in diagnosis of GB polyps. The EUS scoring system based on significant EUS variables including tumor maximum size, internal echo pattern, and hyperechoic spotting has been developed to predict the neoplastic potential of the polyps.413 Higher EUS scores indicate higher probability for neoplastic polyp. Choi et al.13 reported that the risk of neoplastic polyp was significantly higher for polyps with a score of ≥6, as compared to a score of <6. In addition, Sadamoto et al.4 reported that polyps scoring ≥ 12 showed sensitivity, specificity and accuracy for determining the presence of neoplasia of 78%, 83%, and 83%, respectively. Abdominal CT is often incapable of detecting polyps <10 mm; larger polyps appear as soft tissue density projections into the lumen of the bladder, and demonstrate similar enhancement to the rest of the gallbladder. More intense enhancement should be viewed with suspicion, as it is more commonly associated with malignant lesions.1415 Jang et al.16 reported the conventional CT scans' diagnostic sensitivity and specificity for malignant polyp of 72% and 44.4%, respectively.
Thus, EUS is more accurate than abdominal USG in diagnosis of GB polyps, but has low accuracy for differentiating malignant from benign lesions of <1.0 cm.17 However, EUS is associated with discomfort and requires sedation during procedure; and abdominal CT is incapable of detecting low density lesions, and has poor sensitivity for diagnosis of GB polyps, especially in GB polyps <10 mm in diameter, but is useful for studying GB carcinoma, anatomic correlations, and metastases of the ganglia.1819 For these reasons, CT or EUS is used for patients with suspected malignant GB polyp in USG and is not recommended as a first line imaging modality, as compared with abdominal USG. Excluding cholesterol polyps in large size polyps would be the initial approach in cases of malignant polyps. Further evaluation of diagnostic tools, such as CT, EUS and tumor markers is strongly recommended for patients with these polyps. In this study, we characterized the clinical and radiological features of the cholesterol polyp.
Age has a significant association with malignant polyps and is considered an important independent risk factor.62021 Our study showed that the younger mean age (<48.5 years) is an important predictor for cholesterol polyps. The relationship between metabolic syndrome and the development of cholesterol polyps has been previously reported.22223 While we were unable to show any relationship between BMI and cholesterol polyps group, the serum cholesterol level tended to be higher in the cholesterol polyps group, without significant difference.
In view of the maximal diameter of GB polyp measured by abdominal USG, it is well-known that the size of a GB polyp is related to malignancy. Many studies have indicated that GB polyp of >10 mm has a high risk of malignancy and this size is among the criteria for surgical intervention.24252627 The maximal diameter measured on abdominal USG (<13.25 mm) is an important predictor for cholesterol polyps. In another retrospective analysis of 354 subjects with cholecystectomy, the authors suggested that the size criteria for cholecystectomy should be increased from 10 mm to 12 mm.28 Likewise, our result indicated larger size than previous criteria, possibly due to non-inclusion of small polyps of <10 mm. Thus, patients with polyp >10 mm but <13 mm could be diagnosed with cholesterol GB polyp.
Regarding the number of polyps, single polyp is more likely to be a malignant polyp. More aggressive interventions are required for single polyp, as compared to multiple polyp.27 Among our study population, >80% of the non-cholesterol polyps were solitary and about 50% of cholesterol polyps were multiple polyps. Our study also indicated the need for aggressive work-up in solitary polyps as the ratio of solitary lesion is higher in non-cholesterol polyps.
With regards to concurrent GB stone and malignant GB polyp, cholelithiasis associated with GB polyp is a previously reported risk factor.62930 In contrast, no definite relationship was found between the malignant potential of GB polyps and concurrent cholelithiasis. Kwon et al.31 studied 291 patients with confirmed GB polyp on histopathology of specimen obtained from cholecystectomy; of these, 256 patients showed benign GB polyps, 35 patients malignant GB polyps, and associated gallstone in 21.5% (n=55) and 17.1% (n=6) of patients in benign and malignant group, respectively, without statistical significance. Our study showed that the total number of patients with GB polyp and GB stone were low; moreover, no significant difference was found when polyps were related to concurrent GB stone.
Sessile morphology of polyps is a factor for high probability of malignancy.3233 Malignant GB polyps frequently show sessile morphology due to their in situ origin from flat, dysplastic epithelium, present in sessile lesions.34 In our study, the shape of the polypoid lesions was not related to cholesterol polyps group.
Our study had some limitations. First, we were unable to obtain more detailed patient information in all cases, such as the diet habits, family history, occupation and follow-up duration, because this was not a prospective study. Second, the sample size of the study was small. Further studies are needed to identify other risk factors, such as computed tomographic findings, additional sonographic findings and discrepancy between preoperative and postoperative maximum polyp diameters. Multicenter and comparative studies for preoperative predictive factors of cholesterol GB polyp are also necessary.
In conclusion, for the patients with GB polyp of >10 mm maximal diameter on abdominal USG, pre-operative predictive factors of cholesterol polyps included age of <48.5 years, multiple polyps, and polyp size of <13.25 mm. For cases in which these factors are not applicable, it is strongly recommended to evaluate further diagnostic tools in accordance with suspicious malignant GB polyps.
References
1. Chen CY, Lu CL, Chang FY, Lee SD. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol. 1997; 92:2066–2068. PMID: 9362194.
2. Segawa K, Arisawa T, Niwa Y, Suzuki T, Tsukamoto Y, Goto H, et al. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992; 87:630–633. PMID: 1595653.
3. Choi SB, Han HJ, Kim CY, Kim WB, Song TJ, Suh SO, et al. Incidental gallbladder cancer diagnosed following laparoscopic cholecystectomy. World J Surg. 2009; 33:2657–2663. PMID: 19823903.

4. Sadamoto Y, Oda S, Tanaka M, Harada N, Kubo H, Eguchi T, et al. A useful approach to the differential diagnosis of small polypoid lesions of the gallbladder, utilizing an endoscopic ultrasound scoring system. Endoscopy. 2002; 34:959–965. PMID: 12471539.

5. Azuma T, Yoshikawa T, Araida T, Takasaki K. Differential diagnosis of polypoid lesions of the gallbladder by endoscopic ultrasonography. Am J Surg. 2001; 181:65–70. PMID: 11248179.

6. Terzi C, Sökmen S, Seçkin S, Albayrak L, Uğurlu M. Polypoid lesions of the gallbladder: report of 100 cases with special reference to operative indications. Surgery. 2000; 127:622–627. PMID: 10840356.

7. Zielinski MD, Atwell TD, Davis PW, Kendrick ML, Que FG. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg. 2009; 13:19–25. PMID: 18972168.

8. Csendes A, Burgos AM, Csendes P, Smok G, Rojas J. Late follow-up of polypoid lesions of the gallbladder smaller than 10 mm. Ann Surg. 2001; 234:657–660. PMID: 11685029.

9. Ito H, Hann LE, D'Angelica M, Allen P, Fong Y, Dematteo RP, et al. Polypoid lesions of the gallbladder: diagnosis and followup. J Am Coll Surg. 2009; 208:570–575. PMID: 19476792.

10. Kar M, Bhattacharyya U, Laha RN, Saha I, Mukhopadhyay M. Haemobilia due to a large gall bladder polyp. J Indian Med Assoc. 2003; 101:554–555. PMID: 15168995.
11. Damore LJ 2nd, Cook CH, Fernandez KL, Cunningham J, Ellison EC, Melvin WS. Ultrasonography incorrectly diagnoses gallbladder polyps. Surg Laparosc Endosc Percutan Tech. 2001; 11:88–91. PMID: 11330390.

12. Matos AS, Baptista HN, Pinheiro C, Martinho F. Gallbladder polyps: how should they be treated and when? Rev Assoc Med Bras (1992). 2010; 56:318–332. PMID: 20676540.
13. Choi WB, Lee SK, Kim MH, Seo DW, Kim HJ, Kim DI, et al. A new strategy to predict the neoplastic polyps of the gallbladder based on a scoring system using EUS. Gastrointest Endosc. 2000; 52:372–379. PMID: 10968853.

14. Furukawa H, Kosuge T, Shimada K, Yamamoto J, Kanai Y, Mukai K, et al. Small polypoid lesions of the gallbladder: differential diagnosis and surgical indications by helical computed tomography. Arch Surg. 1998; 133:735–739. PMID: 9688001.
15. Ching BH, Yeh BM, Westphalen AC, Joe BN, Qayyum A, Coakley FV. CT differentiation of adenomyomatosis and gallbladder cancer. AJR Am J Roentgenol. 2007; 189:62–66. PMID: 17579153.

16. Jang JY, Kim SW, Lee SE, Hwang DW, Kim EJ, Lee JY, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009; 250:943–949. PMID: 19855259.

17. Cheon YK, Cho WY, Lee TH, Cho YD, Moon JH, Lee JS, et al. Endoscopic ultrasonography does not differentiate neoplastic from non-neoplastic small gallbladder polyps. World J Gastroenterol. 2009; 15:2361–2366. PMID: 19452579.

18. Lee KF, Wong J, Li JC, Lai PB. Polypoid lesions of the gallbladder. Am J Surg. 2004; 188:186–190. PMID: 15249249.

19. Okamoto M, Okamoto H, Kitahara F, Kobayashi K, Karikome K, Miura K, et al. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am J Gastroenterol. 1999; 94:446–450. PMID: 10022644.

20. Yeh CN, Jan YY, Chao TC, Chen MF. Laparoscopic cholecystectomy for polypoid lesions of the gallbladder: a clinicopathologic study. Surg Laparosc Endosc Percutan Tech. 2001; 11:176–181. PMID: 11444747.
21. Jørgensen T, Jensen KH. Polyps in the gallbladder. A prevalence study. Scand J Gastroenterol. 1990; 25:281–286. PMID: 2320947.
22. Sahlin S, Granström L, Gustafsson U, Ståhlberg D, Backman L, Einarsson K. Hepatic esterification rate of cholesterol and biliary lipids in human obesity. J Lipid Res. 1994; 35:484–490. PMID: 8014583.

23. Sandri L, Colecchia A, Larocca A, Vestito A, Capodicasa S, Azzaroli F, et al. Gallbladder cholesterol polyps and cholesterolosis. Minerva Gastroenterol Dietol. 2003; 49:217–224. PMID: 16484961.
24. Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992; 79:227–229. PMID: 1555088.

25. Akatsu T, Aiura K, Shimazu M, Ueda M, Wakabayashi G, Tanabe M, et al. Can endoscopic ultrasonography differentiate nonneoplastic from neoplastic gallbladder polyps? Dig Dis Sci. 2006; 51:416–421. PMID: 16534690.

26. Koga A, Watanabe K, Fukuyama T, Takiguchi S, Nakayama F. Diagnosis and operative indications for polypoid lesions of the gallbladder. Arch Surg. 1988; 123:26–29. PMID: 3276295.

27. Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000; 87:414–417. PMID: 10759734.

28. Park JY, Hong SP, Kim YJ, Kim HJ, Kim HM, Cho JH, et al. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol. 2009; 24:219–222. PMID: 19054258.

29. Bang S. Natural course and treatment strategy of gallbladder polyp. Korean J Gastroenterol. 2009; 53:336–340. PMID: 19556839.

30. Pedersen MR, Dam C, Rafaelsen SR. Ultrasound follow-up for gallbladder polyps less than 6 mm may not be necessary. Dan Med J. 2012; 59:A4503. PMID: 23158888.
31. Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009; 24:481–487. PMID: 19543513.

32. Pejić MA, Milić DJ. Surgical treatment of polypoid lesions of gallbladder. Srp Arh Celok Lek. 2003; 131:319–324. PMID: 14692147.
33. Ishikawa O, Ohhigashi H, Imaoka S, Nakaizumi A, Kitamura T, Sasaki Y, et al. The difference in malignancy between pedunculated and sessile polypoid lesions of the gallbladder. Am J Gastroenterol. 1989; 84:1386–1390. PMID: 2683741.
34. Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH, et al. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008; 2:88–94. PMID: 20485616.

Fig. 2
Reciever-operator characteristic curve of the sonographic size of the polypoid lesions of the gallbladder.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download