INTRODUCTION
MATERIALS AND METHODS
Animals
Induction of retinal degeneration
Tissue preparation
Antibody characterization and immunofluorescence staining
Western blot analysis
Cell counts
Statistical analysis
RESULTS
Degenerative retinal changes after MNU treatment
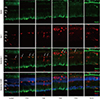 | Fig. 1Nestin and Iba-1 expression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), Iba-1(red), and DAPI (blue) is shown. DAPI was used for nuclei staining to visualize the retinal layers. The decrease in thickness of the ONL due to selective loss of photoreceptor cells was becoming obvious by day 3 after MNU injection. Progressive loss of photoreceptor cells ultimately led to a complete loss of the ONL at day 7 PI. Immunoreactivity of nestin was increased at day 1 PI, peaked at day 3, and declined gradually until day 21. MNU treatment resulted in migrating nestin+/Iba-1+ cells into the ONL. The number of nestin+/Iba-1+ cells peaked at day 3 PI. After that, the number of nestin+/Iba-1+ cells diminished progressively, particularly at day 7, and the cells were sparsely distributed by day 21. Arrows indicate nestin/Iba-1 co-immunolabeling.
Iba-1 = ionized calcium-binding adaptor molecule, DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, MNU = N-methyl-N-nitrosourea, PI = post injection, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
|
Nestin expression in resting retina
Up-regulation of nestin expression after MNU treatment
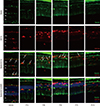 | Fig. 2Nestin and CD11bexpression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), CD11b (red), and DAPI (blue) is shown. Arrows indicate nestin/CD11b co-immunolabeling. After a single injection of MNU, nestin+/CD11b+ cells migrated into the ONL at day 3 and showed a peak at day 5. The number of nestin+/CD11b+ cells diminished progressively, particularly after 7 days, with sparse distribution by day 21.
DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, MNU = N-methyl-N-nitrosourea, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
|
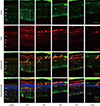 | Fig. 3Nestin and F4/80 expression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), F4/80 (red), and DAPI (blue) is shown. Arrows indicate nestin and F4/80 co-immunolabeling. The number of amoeboid nestin+/F4/80+ cells increased markedly from day 3 to day 5 post injection and decreased thereafter.
DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
|
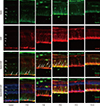 | Fig. 4Nestin and GFAP expression in control and degenerated adult mouse retina.
Immunofluorescent labeling with nestin (green), GFAP (red), and DAPI (blue) is shown. Arrows indicate nestin/GFAP co-immunolabeling. The number of nestin+/GFAP+ Müller cells increased progressively at day 3 after MNU treatment, peaked at day 5, and decreased thereafter. GFAP-positive cells remained in an activated state at day 7 and day 21 post injection, despite the diminution in retinal thickness.
GFAP = glial fibrillary acidic protein, DAPI = 4-6-diamino-2-phenylindole, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer.
|
Western blot analysis
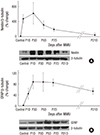 | Fig. 5Western blot analyses of nestin and GFAP expression.
(A) Significantly increased immune-reactivity of nestin at day 1 and significantly decreased to mostly the level of control group at 21 days after MNU injection. (B) Immune-reactivity of GFAP showed gradually increasing for 3 days after MNU injection, and declined at 21 days. Nevertheless, GFAP remained in activated state compared to control group even after 21 days after MNU injection.
GFAP = glial fibrillary acidic protein, MNU = N-methyl-N-nitrosourea.
|
Cell count
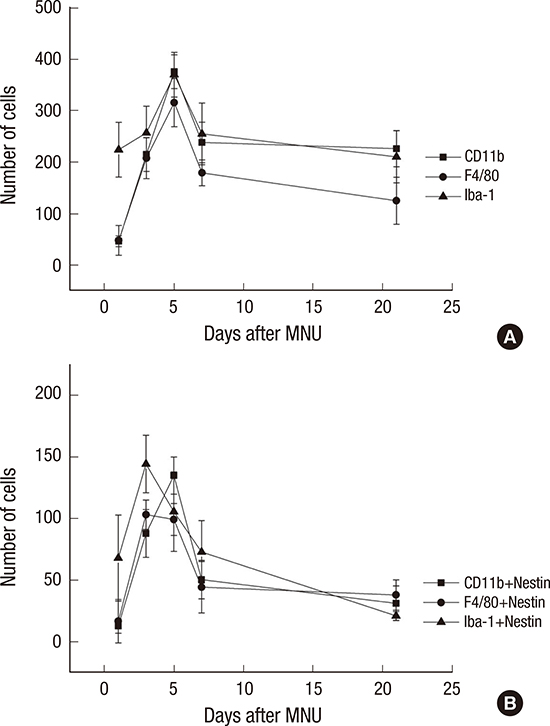
 | Fig. 6Cell counts of Iba-1+, CD11b+, F4/80+, and nestin+ cells.
(A) Iba-1+, CD11b+, F4/80+ cells were peaked at 5 days after injection. (B) Nestin/Iba-1 co-immunolabelling cells and Nestin/F4/80 co-immunolabelling cells were peaked at 3 days after injection. Nestin/CD11b co-immunolabelling cells were peaked at 5 days after injection.
Iba-1 = ionized calcium-binding adaptor molecule, MNU = N-methyl-N-nitrosourea.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download